Organization and Plasticity of Sodium Channel Expression in the Mouse Olfactory and Vomeronasal Epithelia
- PMID: 28420967
- PMCID: PMC5376585
- DOI: 10.3389/fnana.2017.00028
Organization and Plasticity of Sodium Channel Expression in the Mouse Olfactory and Vomeronasal Epithelia
Abstract
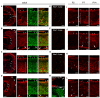
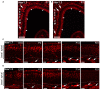
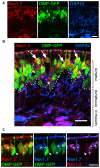
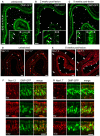
To understand the molecular basis of neuronal excitation in the mammalian olfactory system, we conducted a systematic analysis of the organization of voltage-gated sodium (Nav) channel subunits in the main olfactory epithelium (MOE) and vomeronasal organ (VNO) of adult mice. We also analyzed changes in Nav channel expression during development in these two systems and during regeneration of the MOE. Quantitative PCR shows that Nav1.7 is the predominant isoform in both adult MOE and VNO. We detected pronounced immunoreactivity for Nav1.7 and Nav1.3 in axons of olfactory and vomeronasal sensory neurons (VSNs). Analysis of Nav1.2 and Nav1.6 revealed an unexpected subsystem-specific distribution. In the MOE, these Nav channels are absent from olfactory sensory neurons (OSNs) but present in non-neuronal olfactory cell types. In the VNO, Nav1.2 and Nav1.6 are confined to VSNs, with Nav1.2-immunoreactive somata solely present in the basal layer of the VNO. The subcellular localization of Nav1.3 and Nav1.7 in OSNs can change dramatically during periods of heightened plasticity in the MOE. During the first weeks of development and during regeneration of the olfactory epithelium following chemical lesion, expression of Nav1.3 and Nav1.7 is transiently enhanced in the somata of mature OSNs. Our results demonstrate a highly complex organization of Nav channel expression in the mouse olfactory system, with specific commonalities but also differences between the MOE and the VNO. On the basis of their subcellular localization, Nav1.3 and Nav1.7 should play major roles in action potential propagation in both MOE and VNO, whereas Nav1.2 and Nav1.6 are specific to the function of VSNs. The plasticity of Nav channel expression in OSNs during early development and recovery from injury could reflect important physiological requirements in a variety of activity-dependent mechanisms.
Keywords: development; olfactory epithelium; regeneration; voltage-gated sodium channels; vomeronasal organ.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

