Heme as a Target for Therapeutic Interventions
- PMID: 28420988
- PMCID: PMC5378770
- DOI: 10.3389/fphar.2017.00146
Heme as a Target for Therapeutic Interventions
Abstract
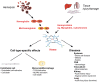
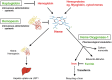
Heme is a complex of iron and the tetrapyrrole protoporphyrin IX with essential functions in aerobic organisms. Heme is the prosthetic group of hemoproteins such as hemoglobin and myoglobin, which are crucial for reversible oxygen binding and transport. By contrast, high levels of free heme, which may occur in various pathophysiological conditions, are toxic via pro-oxidant, pro-inflammatory and cytotoxic effects. The toxicity of heme plays a major role for the pathogenesis of prototypical hemolytic disorders including sickle cell disease and malaria. Moreover, there is increasing appreciation that detrimental effects of heme may also be critically involved in diseases, which usually are not associated with hemolysis such as severe sepsis and atherosclerosis. In mammalians homeostasis of heme and its potential toxicity are primarily controlled by two physiological systems. First, the scavenger protein hemopexin (Hx) non-covalently binds extracellular free heme with high affinity and attenuates toxicity of heme in plasma. Second, heme oxygenases (HOs), in particular the inducible HO isozyme, HO-1, can provide antioxidant cytoprotection via enzymatic degradation of intracellular heme. This review summarizes current knowledge on the pathophysiological role of heme for various diseases as demonstrated in experimental animal models and in humans. The functional significance of Hx and HOs for the regulation of heme homeostasis is highlighted. Finally, the therapeutic potential of pharmacological strategies that apply Hx and HO-1 in various clinical settings is discussed.
Keywords: heme; heme oxygenases; heme toxicity; hemolysis; hemopexin; inflammation; inflammatory diseases.
Figures
Similar articles
-
Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases.Circulation. 2013 Mar 26;127(12):1317-29. doi: 10.1161/CIRCULATIONAHA.112.130179. Epub 2013 Feb 27. Circulation. 2013. PMID: 23446829
-
Role of hemoglobin/heme scavenger protein hemopexin in atherosclerosis and inflammatory diseases.Curr Opin Lipidol. 2015 Oct;26(5):384-7. doi: 10.1097/MOL.0000000000000208. Curr Opin Lipidol. 2015. PMID: 26339767 Free PMC article. Review.
-
Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease.Blood. 2016 Jan 28;127(4):473-86. doi: 10.1182/blood-2015-08-663245. Epub 2015 Dec 16. Blood. 2016. PMID: 26675351 Free PMC article.
-
The hemoglobin degradation pathway in patients with preeclampsia - Fetal hemoglobin, heme, heme oxygenase-1 and hemopexin - Potential diagnostic biomarkers?Pregnancy Hypertens. 2018 Oct;14:273-278. doi: 10.1016/j.preghy.2018.02.005. Epub 2018 Feb 15. Pregnancy Hypertens. 2018. PMID: 29530745
-
Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development.Front Physiol. 2014 Oct 28;5:415. doi: 10.3389/fphys.2014.00415. eCollection 2014. Front Physiol. 2014. PMID: 25389409 Free PMC article. Review.
Cited by
-
Heme Proteins and Kidney Injury: Beyond Rhabdomyolysis.Kidney360. 2022 Oct 5;3(11):1969-1979. doi: 10.34067/KID.0005442022. eCollection 2022 Nov 24. Kidney360. 2022. PMID: 36514409 Free PMC article. Review.
-
Untargeted metabolomics identifies the potential role of monocarboxylate transporter 6 (MCT6/SLC16A5) in lipid and amino acid metabolism pathways.Pharmacol Res Perspect. 2022 Jun;10(3):e00944. doi: 10.1002/prp2.944. Pharmacol Res Perspect. 2022. PMID: 35466588 Free PMC article.
-
Identifying genetic variants underlying medication-induced osteonecrosis of the jaw in cancer and osteoporosis: a case control study.J Transl Med. 2019 Nov 20;17(1):381. doi: 10.1186/s12967-019-2129-3. J Transl Med. 2019. PMID: 31747953 Free PMC article.
-
Pigment Nephropathy: Novel Insights into Inflammasome-Mediated Pathogenesis.Int J Mol Sci. 2019 Apr 23;20(8):1997. doi: 10.3390/ijms20081997. Int J Mol Sci. 2019. PMID: 31018590 Free PMC article. Review.
-
Vitamin B₆ and Its Role in Cell Metabolism and Physiology.Cells. 2018 Jul 22;7(7):84. doi: 10.3390/cells7070084. Cells. 2018. PMID: 30037155 Free PMC article. Review.
References
-
- Abraham N. G., Friedland M. L., Lever R. (1983). Heme metabolism in hepatic and erythroid cells, in Progress in Hematology, ed Brown E. (New York: Grune and Stratton; ), 75–130. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

