FDA Facilitated Regulatory Pathways: Visualizing Their Characteristics, Development, and Authorization Timelines
- PMID: 28420989
- PMCID: PMC5376616
- DOI: 10.3389/fphar.2017.00161
FDA Facilitated Regulatory Pathways: Visualizing Their Characteristics, Development, and Authorization Timelines
Abstract
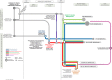
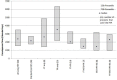
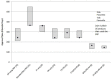
The US Food and Drug Administration (FDA) has four facilitated regulatory pathways (FRPs): Fast Track (FT), Breakthrough Therapy (BTD), Priority Review (PR), and Accelerated Approval (AA). Only PR specifies an expedited review timeline (6 months). We sought to determine to what extent the combination of two or more FRPs influenced development and approval times. We developed a "metro map" to illustrate FRP elements and their influence on review times. We assessed 125 new active substances (approved January 2013 to December 2015) 74 of which used one or more FRPs. For these 74, development times ranged from 1,458 (BTD + PR + AA) to 3,515 days (PR). PR alone had a median approval time of 242 days. The most common combination was FT + PR (median approval 292 days, n = 21). The fastest approval times were for PR + FT + BTD + AA (145 days) and PR + BTD + AA (166 days). Our findings support the combination of FRPs for shortening development and review times beyond that provided by PR alone.
Keywords: Accelerated Approval; Breakthrough Therapy; FDA; Fast Track; Priority Review; facilitated regulatory pathway; review times.
Figures
References
-
- Bujar M., McAuslane N., Liberti L. (2015). New Drug Approvals in ICH Countries 2005 – 2014. Focus on Facilitated Regulatory Pathways and Orphan Designations CIRS R& D Briefing 57. Available at: http://www.cirsci.org/sites/default/files/CIRS_R&D_57_ICH_%20approval_%2...
-
- FDA Guidance for Industry (2014). Expedited Programs for Serious Conditions – Drugs and Biologics. Washington, DC: U.S. Department of Health and Human Services.
-
- GAO Report (2015). Drug Safety- FDA Expedites Many Applications, but Data for Post-Approval Oversight Need Improvement. GAO-16-192. Available at: http://www.gao.gov/products/GAO-16-192
-
- Kalesnik-Orszulak R., Dixit D., Boulos D., Toscani M., Barone J. A. (2016). Effect of Breakthrough Therapy Designation on Marketing Application Review 2013–2015. Hamburg: EuroDIA.
-
- Liberti L., Breckenridge A., Hoekman J., Leufkens H., Lumpkin M., McAuslane N., et al. (2016). Accelerating access to new medicines: current status of facilitated regulatory pathways used by emerging regulatory authorities. J. Public Health Policy 10.1057/jphp.2016.8 [Epub ahead of print]. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous