MicroRNAs in Palatogenesis and Cleft Palate
- PMID: 28420997
- PMCID: PMC5378724
- DOI: 10.3389/fphys.2017.00165
MicroRNAs in Palatogenesis and Cleft Palate
Abstract
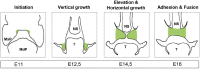
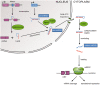
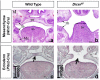
Palatogenesis requires a precise spatiotemporal regulation of gene expression, which is controlled by an intricate network of transcription factors and their corresponding DNA motifs. Even minor perturbations of this network may cause cleft palate, the most common congenital craniofacial defect in humans. MicroRNAs (miRNAs), a class of small regulatory non-coding RNAs, have elicited strong interest as key regulators of embryological development, and as etiological factors in disease. MiRNAs function as post-transcriptional repressors of gene expression and are therefore able to fine-tune gene regulatory networks. Several miRNAs are already identified to be involved in congenital diseases. Recent evidence from research in zebrafish and mice indicates that miRNAs are key factors in both normal palatogenesis and cleft palate formation. Here, we provide an overview of recently identified molecular mechanisms underlying palatogenesis involving specific miRNAs, and discuss how dysregulation of these miRNAs may result in cleft palate.
Keywords: cleft palate; genetics; miRNA; palatogenesis; post-transcriptional regulation.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

