The Synergistic Responses of Different Photoprotective Pathways in Dwarf Bamboo (Fargesia rufa) to Drought and Subsequent Rewatering
- PMID: 28421106
- PMCID: PMC5378818
- DOI: 10.3389/fpls.2017.00489
The Synergistic Responses of Different Photoprotective Pathways in Dwarf Bamboo (Fargesia rufa) to Drought and Subsequent Rewatering
Abstract
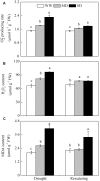
Dwarf bamboo-dominated forests are often subjected to temporary periods of drought due to rising air temperature and decreasing rainfall. Nevertheless, the relationship among CO2 assimilation, photoprotective pathways and metabolism of reactive oxygen species (ROS) remains unexplored in bamboo species. Changes in leaf gas exchange, chlorophyll fluorescence, energy partitioning, antioxidative system and compounds related to ROS metabolism in Fargesia rufa plants subjected to drought and subsequent rewatering were analyzed. Drought resulted in a reversible inhibition of photochemistry, particularly net CO2 assimilation, and lipid peroxidation due to ROS accumulation. Meanwhile, photoprotective pathways, including the water-water cycle (especially for moderate drought), and adjustment in antenna pigments, thermal dissipation and antioxidative defense capacity at organelle levels (especially for severe drought), were up-regulated at the stress phase. Conversely, photorespiration was down-regulated after drought stress. As a result, rewatering restored most of the photochemical activity under drought, especially moderate drought. Moreover, thermal dissipation under severe drought was still operated for avoiding high ROS levels after rewatering. Therefore, the synergistic function of these photoprotective pathways except photorespiration can protect the photosynthetic apparatus from oxidative damage in response to varying intensities of drought stress when CO2 assimilation is restricted. This is helpful for the gradual recovery of photosynthetic capacity after rewatering. Thus, F. rufa plants can withstand drought and is capable of survival in such environment.
Highlights: 1. The effects of drought and subsequent rewatering on Fargesia rufa were studied.2. Drought resulted in a reversible inhibition of photochemistry.3. Photoprotective pathways except photorespiration were up-regulated at the drought phase.4. Rewatering rapidly restored photochemical activity, especially under moderate drought.5. Fargesia rufa plant is capable of resisting and surviving drought environment.
Keywords: CO2 assimilation; antioxidative defense system; energy partitioning; rewatering; the water–water cycle; thermal dissipation.
Figures





Similar articles
-
Photoprotective and antioxidative mechanisms against oxidative damage in Fargesia rufa subjected to drought and salinity.Funct Plant Biol. 2017 Feb;44(3):302-311. doi: 10.1071/FP16214. Funct Plant Biol. 2017. PMID: 32480565
-
Photoprotection regulated by phosphorus application can improve photosynthetic performance and alleviate oxidative damage in dwarf bamboo subjected to water stress.Plant Physiol Biochem. 2017 Sep;118:88-97. doi: 10.1016/j.plaphy.2017.05.022. Epub 2017 Jun 8. Plant Physiol Biochem. 2017. PMID: 28624684
-
Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery.New Phytol. 2007;174(4):799-810. doi: 10.1111/j.1469-8137.2007.02047.x. New Phytol. 2007. PMID: 17504463
-
Plant responses to drought and rewatering.Plant Signal Behav. 2010 Jun;5(6):649-54. doi: 10.4161/psb.5.6.11398. Epub 2010 Jun 1. Plant Signal Behav. 2010. PMID: 20404516 Free PMC article. Review.
-
Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts.J Plant Physiol. 2016 Sep 20;203:84-94. doi: 10.1016/j.jplph.2016.04.002. Epub 2016 Apr 7. J Plant Physiol. 2016. PMID: 27083537 Review.
Cited by
-
Osmotic Adjustment and Antioxidant System Regulated by Nitrogen Deposition Improve Photosynthetic and Growth Performance and Alleviate Oxidative Damage in Dwarf Bamboo Under Drought Stress.Front Plant Sci. 2022 Apr 14;13:819071. doi: 10.3389/fpls.2022.819071. eCollection 2022. Front Plant Sci. 2022. PMID: 35498701 Free PMC article.
-
Mitochondrial Biogenesis in Diverse Cauliflower Cultivars under Mild and Severe Drought. Impaired Coordination of Selected Transcript and Proteomic Responses, and Regulation of Various Multifunctional Proteins.Int J Mol Sci. 2018 Apr 10;19(4):1130. doi: 10.3390/ijms19041130. Int J Mol Sci. 2018. PMID: 29642585 Free PMC article.
-
Comparative Analyses of Anatomical Structure, Phytohormone Levels, and Gene Expression Profiles Reveal Potential Dwarfing Mechanisms in Shengyin Bamboo (Phyllostachys edulis f. tubaeformis).Int J Mol Sci. 2018 Jun 7;19(6):1697. doi: 10.3390/ijms19061697. Int J Mol Sci. 2018. PMID: 29875341 Free PMC article.
-
Incorporation of compost and biochar enhances yield and medicinal compounds in seeds of water-stressed Trigonella foenum-graecum L. plants cultivated in saline calcareous soils.BMC Plant Biol. 2024 Jun 12;24(1):538. doi: 10.1186/s12870-024-05182-6. BMC Plant Biol. 2024. PMID: 38867179 Free PMC article.
-
Unveiling the Hidden Responses: Metagenomic Insights into Dwarf Bamboo (Fargesia denudata) Rhizosphere under Drought and Nitrogen Challenges.Int J Mol Sci. 2024 Oct 8;25(19):10790. doi: 10.3390/ijms251910790. Int J Mol Sci. 2024. PMID: 39409119 Free PMC article.
References
-
- Arrigoni O., Dipierro S., Borraccino G. (1981). Ascorbate free radical reductase, a key enzyme of the ascorbic acid system. FEBS Lett. 125 242–244. 10.1016/0014-5793(81)80729-6 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

