Changes in Composition of the Gut Bacterial Microbiome after Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in a Pediatric Heart Transplant Patient
- PMID: 28421185
- PMCID: PMC5378704
- DOI: 10.3389/fcvm.2017.00017
Changes in Composition of the Gut Bacterial Microbiome after Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in a Pediatric Heart Transplant Patient
Abstract
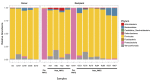
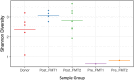
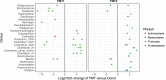
The microbiome is increasingly recognized as an important influence on human health and many of the comorbidities that affect patients after solid organ transplantation (SOT) have been shown to involve changes in gut bacterial populations. Thus, microbiome changes in an individual patient may have important health implications after SOT but this area remains understudied. We describe changes in the composition of the fecal microbiome from a pediatric heart transplant recipient before and >2.5 years after he underwent repeated fecal microbiota transplantation (FMT) for recurrent Clostridium difficile infection (CDI). With both documented episodes of CDI, there was marked loss of bacterial diversity with overgrowth of Proteobacteria (>98.9% of phyla identified) associated with symptomatic colitis that was corrected after FMT. We hypothesize that a second CDI occurring after FMT was related to incomplete restoration of normal bowel flora post-FMT with relative deficiencies of the phyla Firmicutes and Bacteroidetes and the families Lachnospiraceae and Ruminococcaceae. Following the second FMT, there was a gradual shift in gut bacterial composition coincident with the recipient developing lymphonodular hyperplasia of the colon and painless hematochezia that resolved with discontinuation of mycophenolate mofetil (MMF). This case documents dynamic changes in the bacterial microbiome after FMT and suggests that MMF may influence the gut microbiome with consequences for the patient.
Keywords: fecal microbiota transplant; heart transplantation; immunosuppression; microbiome; pediatric.
Figures
Similar articles
-
Longitudinal microbiome analysis of single donor fecal microbiota transplantation in patients with recurrent Clostridium difficile infection and/or ulcerative colitis.PLoS One. 2018 Jan 31;13(1):e0190997. doi: 10.1371/journal.pone.0190997. eCollection 2018. PLoS One. 2018. PMID: 29385143 Free PMC article.
-
Successful therapy of Clostridium difficile infection with fecal microbiota transplantation.J Physiol Pharmacol. 2016 Dec;67(6):859-866. J Physiol Pharmacol. 2016. PMID: 28195066
-
Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease.Aliment Pharmacol Ther. 2015 Sep;42(6):741-52. doi: 10.1111/apt.13326. Epub 2015 Jul 21. Aliment Pharmacol Ther. 2015. PMID: 26198180
-
Fecal microbiota transplantation for the treatment of Clostridium difficile infection.J Hosp Med. 2016 Jan;11(1):56-61. doi: 10.1002/jhm.2449. Epub 2015 Sep 7. J Hosp Med. 2016. PMID: 26344412 Free PMC article. Review.
-
Fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with solid organ transplants: an institutional experience and review of the literature.Transpl Infect Dis. 2018 Dec;20(6):e12967. doi: 10.1111/tid.12967. Epub 2018 Jul 31. Transpl Infect Dis. 2018. PMID: 30011107 Review.
Cited by
-
Association Between Immunosuppressive Therapy and Outcome of Clostridioides difficile Infection: Systematic Review and Meta-Analysis.Dig Dis Sci. 2022 Aug;67(8):3890-3903. doi: 10.1007/s10620-021-07229-2. Epub 2021 Sep 23. Dig Dis Sci. 2022. PMID: 34554365
-
The Impact of Human Microbiotas in Hematopoietic Stem Cell and Organ Transplantation.Front Immunol. 2022 Jul 7;13:932228. doi: 10.3389/fimmu.2022.932228. eCollection 2022. Front Immunol. 2022. PMID: 35874759 Free PMC article. Review.
-
Exploration of the relationship between intestinal flora changes and gut acute graft-versus-host disease after hematopoietic stem cell transplantation.Transl Pediatr. 2021 Feb;10(2):283-295. doi: 10.21037/tp-20-208. Transl Pediatr. 2021. PMID: 33708514 Free PMC article.
-
Fecal microbiota transplantation for refractory diarrhea in immunocompromised diseases: a pediatric case report.Ital J Pediatr. 2019 Aug 28;45(1):116. doi: 10.1186/s13052-019-0708-9. Ital J Pediatr. 2019. PMID: 31462301 Free PMC article.
-
Clostridioides difficile Infections: Prevention and Treatment Strategies.Adv Exp Med Biol. 2024;1449:175-186. doi: 10.1007/978-3-031-58572-2_11. Adv Exp Med Biol. 2024. PMID: 39060738 Review.
References
-
- Tourret J, Willing BP, Dion S, MacPherson J, Denamur E, Finlay BB. Immunosuppressive treatment alters secretion of ileal antimicrobial peptides and gut microbiota, and favors subsequent colonization by uropathogenic Escherichia coli. Transplantation (2017) 101(1):74–82. 10.1097/TP.0000000000001503 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

