Role of mucosa in generating spontaneous activity in the guinea pig seminal vesicle
- PMID: 28421606
- PMCID: PMC5509876
- DOI: 10.1113/JP273872
Role of mucosa in generating spontaneous activity in the guinea pig seminal vesicle
Abstract
Key points: The mucosa may have neuron-like functions as urinary bladder mucosa releases bioactive substances that modulate sensory nerve activity as well as detrusor muscle contractility. However, such mucosal function in other visceral organs remains to be established. The role of mucosa in generating spontaneous contractions in seminal vesicles (SVs), a paired organ in the male reproductive tract, was investigated. The intact mucosa is essential for the generation of spontaneous phasic contractions of SV smooth muscle arising from electrical slow waves and corresponding increases in intracellular Ca2+ . These spontaneous events primarily depend on Ca2+ handling by sarco-endoplasmic reticulum Ca2+ stores. A population of mucosal cells developed spontaneous rises in intracellular Ca2+ relying on sarco-endoplasmic reticulum Ca2+ handling. The spontaneously active cells in the SV mucosa appear to drive spontaneous activity in smooth muscle either by sending depolarizing signals and/or by releasing humoral substances.
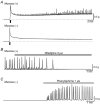
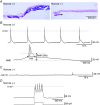
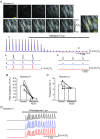
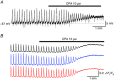
Abstract: The role of the mucosa in generating the spontaneous activity of guinea-pig seminal vesicle (SV) was explored. Changes in contractility, membrane potential and intracellular Ca2+ dynamics of SV smooth muscle cells (SMCs) were recorded using isometric tension recording, intracellular microelectrode recording and epi-fluorescence Ca2+ imaging, respectively. Mucosa-intact but not mucosa-denuded SV preparations generated TTX- (1 μm) resistant spontaneous phasic contractions that were abolished by nifedipine (3 μm). Consistently, SMCs developed mucosa-dependent slow waves (SWs) that triggered action potentials and corresponding Ca2+ flashes. Nifedipine (10 μm) abolished the action potentials and spontaneous contractions, while suppressing the SWs and Ca2+ flashes. Both the residual SWs and spontaneous Ca2+ transients were abolished by cyclopiazonic acid (CPA, 10 μm), a sarco-endoplasmic reticulum Ca2+ -ATPase (SERCA) inhibitor. DIDS (300 μm) and niflumic acid (100 μm), blockers for Ca2+ -activated Cl- channels (CACCs), or low Cl- solution also slowed or prevented the generation of SWs. In SV mucosal preparations detached from the muscle layer, a population of mucosal cells generated spontaneous Ca2+ transients that were blocked by CPA but not nifedipine. These results suggested that spontaneous contractions and corresponding Ca2+ flashes in SV SMCs arise from action potential generation due to the opening of L-type voltage-dependent Ca2+ channels. Spontaneous Ca2+ transients appear to primarily result from Ca2+ release from sarco-endoplasmic reticulum Ca2+ stores to activate CACCs to develop SWs. The mucosal cells firing spontaneous Ca2+ transients may play a critical role in driving spontaneous activity of SV smooth muscle either by sending depolarizing signals or by releasing humoral substances.
Keywords: intracellular Ca2+ release; mucosa; seminal vesicle; slow wave; spontaneous contraction.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures






Comment in
-
A seminal study on the mechanisms underlying spontaneous activity of the seminal vesicles?J Physiol. 2017 Jul 15;595(14):4567. doi: 10.1113/JP274499. Epub 2017 Jun 1. J Physiol. 2017. PMID: 28488278 Free PMC article. No abstract available.
Similar articles
-
Mucosa-Dependent, Stretch-Sensitive Spontaneous Activity in Seminal Vesicle.Adv Exp Med Biol. 2019;1124:217-231. doi: 10.1007/978-981-13-5895-1_9. Adv Exp Med Biol. 2019. PMID: 31183829 Review.
-
PDGFRα+ subepithelial interstitial cells act as a pacemaker to drive smooth muscle of the guinea pig seminal vesicle.J Physiol. 2022 Apr;600(7):1703-1730. doi: 10.1113/JP281686. Epub 2022 Feb 25. J Physiol. 2022. PMID: 35081665
-
Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder.Br J Pharmacol. 2004 Jan;141(1):183-93. doi: 10.1038/sj.bjp.0705602. Epub 2003 Dec 8. Br J Pharmacol. 2004. PMID: 14662721 Free PMC article.
-
Heterogeneous CPA sensitivity of spontaneous excitation in smooth muscle of the rabbit urethra.Br J Pharmacol. 2006 Jun;148(3):340-9. doi: 10.1038/sj.bjp.0706729. Br J Pharmacol. 2006. PMID: 16582935 Free PMC article.
-
Role of Pericytes in the Initiation and Propagation of Spontaneous Activity in the Microvasculature.Adv Exp Med Biol. 2019;1124:329-356. doi: 10.1007/978-981-13-5895-1_14. Adv Exp Med Biol. 2019. PMID: 31183834 Review.
Cited by
-
A seminal study on the mechanisms underlying spontaneous activity of the seminal vesicles?J Physiol. 2017 Jul 15;595(14):4567. doi: 10.1113/JP274499. Epub 2017 Jun 1. J Physiol. 2017. PMID: 28488278 Free PMC article. No abstract available.
-
Identification of PDGFRα-positive interstitial cells in the distal segment of the murine vas deferens.Sci Rep. 2021 Apr 6;11(1):7553. doi: 10.1038/s41598-021-87049-6. Sci Rep. 2021. PMID: 33824385 Free PMC article.
-
Insights on Platelet-Derived Growth Factor Receptor α-Positive Interstitial Cells in the Male Reproductive Tract.Int J Mol Sci. 2024 Apr 8;25(7):4128. doi: 10.3390/ijms25074128. Int J Mol Sci. 2024. PMID: 38612936 Free PMC article. Review.
References
-
- Aumüller G & Riva A (1992). Morphology and functions of the human seminal vesicle. Andrologia 24, 183–196. - PubMed
-
- Birowo P, Ückert S, Kedia GT, Sonnenberg JE, Sandner P, Thon WF, Scheller F, Rahardjo D & Kuczyk MA (2010). Exposure of human seminal vesicle tissue to phosphodiesterase (PDE) inhibitors antagonizes the contraction induced by norepinephrine and increases production of cyclic nucleotides. Urology 76, 1518.e1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

