Cloning and plant-based production of antibody MC10E7 for a lateral flow immunoassay to detect [4-arginine]microcystin in freshwater
- PMID: 28421663
- PMCID: PMC5785354
- DOI: 10.1111/pbi.12746
Cloning and plant-based production of antibody MC10E7 for a lateral flow immunoassay to detect [4-arginine]microcystin in freshwater
Abstract
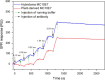
Antibody MC10E7 is one of a small number of monoclonal antibodies that bind specifically to [Arg4]-microcystins, and it can be used to survey natural water sources and food samples for algal toxin contamination. However, the development of sensitive immunoassays in different test formats, particularly user-friendly tests for on-site analysis, requires a sensitive but also cost-effective antibody. The original version of MC10E7 was derived from a murine hybridoma, but we determined the sequence of the variable regions using the peptide mass-assisted cloning strategy and expressed a scFv (single-chain variable fragment) format of this antibody in yeast and a chimeric full-size version in leaves of Nicotiana tabacum and Nicotiana benthamiana to facilitate inexpensive and scalable production. The specific antigen-binding activity of the purified antibody was verified by surface plasmon resonance spectroscopy and ELISA, confirming the same binding specificity as its hybridoma-derived counterpart. The plant-derived antibody was used to design a lateral flow immunoassay (dipstick) for the sensitive detection of [Arg4]-microcystins at concentrations of 100-300 ng/L in freshwater samples collected at different sites. Plant-based production will likely reduce the cost of the antibody, currently the most expensive component of the dipstick immunoassay, and will allow the development of further antibody-based analytical devices and water purification adsorbents for the efficient removal of toxic contaminants.
Keywords: lateral flow immunoassay; microcystin; molecular farming; plant-made antibody; water contamination.
© 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures



References
-
- Barbi, T. , Drake, P.M. , Drever, M. , van Dolleweerd, C.J. , Porter, A.R. and Ma, J.K. (2011) Generation of transgenic plants expressing plasma membrane‐bound antibodies to the environmental pollutant microcystin‐LR. Transgenic Res. 20, 701–707. - PubMed
-
- Bechtold, N. and Pelletier, G. (1998) In planta Agrobacterium‐mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82, 259–266. - PubMed
-
- Bendandi, M. , Marillonnet, S. , Kandzia, R. , Thieme, F. , Nickstadt, A. , Herz, S. , Frode, R. et al. (2010) Rapid, high‐yield production in plants of individualized idiotype vaccines for non‐Hodgkin's lymphoma. Ann. Oncol. 21, 2420–2427. - PubMed
-
- Bialczyk, J. , Kochanowski, A. , Czaja‐Prokop, U. and Chrapusta, E. (2014) Removal of microcystin‐LR from water by polymers based on N‐vinylformamide structure. Water Sci. Technol.: Water Supply, 14, 230–237.
-
- Bird, R.E. and Walker, B.W. (1991) Single chain antibody variable regions. Trends Biotechnol. 9, 132–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

