Small serine recombination systems ParA-MRS and CinH-RS2 perform precise excision of plastid DNA
- PMID: 28421718
- PMCID: PMC5698047
- DOI: 10.1111/pbi.12740
Small serine recombination systems ParA-MRS and CinH-RS2 perform precise excision of plastid DNA
Abstract
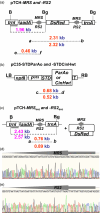
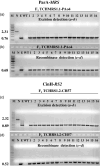
Selectable marker genes (SMGs) are necessary for selection of transgenic plants. However, once stable transformants have been identified, the marker gene is no longer needed. In this study, we demonstrate the use of the small serine recombination systems, ParA-MRS and CinH-RS2, to precisely excise a marker gene from the plastid genome of tobacco. Transplastomic plants transformed with the pTCH-MRS and pTCH-RS2 vectors, containing the visual reporter gene DsRed flanked by directly oriented MRS and RS2 recognition sites, respectively, were crossed with nuclear-genome transformed tobacco plants expressing plastid-targeted ParA and CinH recombinases, respectively. One hundred per cent of both types of F1 hybrids exhibited excision of the DsRed marker gene. PCR and Southern blot analyses of DNA from F2 plants showed that approximately 30% (CinH-RS2) or 40% (ParA-MRS) had lost the recombinase genes by segregation. The postexcision transformed plastid genomes were stable and the excision events heritable. The ParA-MRS and CinH-RS2 recombination systems will be useful tools for site-specific manipulation of the plastid genome and for generating marker-free plants, an essential step for reuse of SMG and for addressing concerns about the presence of antibiotic resistance genes in transgenic plants.
Keywords: CinH-RS2; ParA-MRS; marker excision; site-specific recombination.
© 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Precise excision of plastid DNA by the large serine recombinase Bxb1.Plant Biotechnol J. 2014 Apr;12(3):322-9. doi: 10.1111/pbi.12139. Epub 2013 Nov 22. Plant Biotechnol J. 2014. PMID: 24261912
-
Transgene excision in pollen using a codon optimized serine resolvase CinH-RS2 site-specific recombination system.Plant Mol Biol. 2011 Apr;75(6):621-31. doi: 10.1007/s11103-011-9756-2. Epub 2011 Feb 26. Plant Mol Biol. 2011. PMID: 21359553
-
Plastid marker gene excision by the phiC31 phage site-specific recombinase.Plant Mol Biol. 2007 May;64(1-2):137-43. doi: 10.1007/s11103-007-9140-4. Epub 2007 Feb 9. Plant Mol Biol. 2007. PMID: 17294253
-
Construction of marker-free transplastomic plants.Curr Opin Biotechnol. 2007 Apr;18(2):107-14. doi: 10.1016/j.copbio.2007.02.003. Epub 2007 Mar 6. Curr Opin Biotechnol. 2007. PMID: 17339108 Review.
-
Advances of selectable marker genes in plastid genetic engineering.Yi Chuan. 2017 Sep 20;39(9):810-827. doi: 10.16288/j.yczz.16-433. Yi Chuan. 2017. PMID: 28936979 Review.
Cited by
-
Plastid Marker Gene Excision in the Tobacco Shoot Apex by Agrobacterium-Delivered Cre Recombinase.Methods Mol Biol. 2021;2317:177-193. doi: 10.1007/978-1-0716-1472-3_9. Methods Mol Biol. 2021. PMID: 34028769
-
Transgene Bioconfinement: Don't Flow There.Plants (Basel). 2023 Mar 1;12(5):1099. doi: 10.3390/plants12051099. Plants (Basel). 2023. PMID: 36903958 Free PMC article. Review.
-
Marker-Free Transplastomic Plants by Excision of Plastid Marker Genes Using Directly Repeated DNA Sequences.Methods Mol Biol. 2021;2317:95-107. doi: 10.1007/978-1-0716-1472-3_4. Methods Mol Biol. 2021. PMID: 34028764
References
-
- Ahmad, A. , Pereira, E.O. , Conley, A.J. , Richman, A.S. and Menassa, R. (2010) Green biofactories: recombinant protein production in plants. Recent Patents Biotechnol. 4, 242–259. - PubMed
-
- Ballester, A. , Cervera, M. and Pena, L. (2006) Efficient production of transgenic citrus plants using isopentenyl transferase positive selection and removal of the marker gene by site‐specific recombination. Plant Cell Rep. 26, 39–44. - PubMed
-
- Bevis, B.J. and Glick, B.S. (2002) Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat. Biotech. 20, 83–87. - PubMed
-
- Blechl, A.E. , Lin, J.W. , Shao, M. , Thilmony, R.L. and Thomson, J.G. (2012) Bxb1 recombinase mediates site‐specific deletions in transgenic wheat. Plant Mol. Biol. Rep. 30, 1357–1366.
-
- Bock, R. (2015) Engineering plastid genomes: methods, tools, and applications in basic research and biotechnology. Annu. Rev. Plant Biol. 66, 211–241. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

