Cell Origin Dictates Programming of Resident versus Recruited Macrophages during Acute Lung Injury
- PMID: 28421818
- PMCID: PMC5625228
- DOI: 10.1165/rcmb.2017-0061OC
Cell Origin Dictates Programming of Resident versus Recruited Macrophages during Acute Lung Injury
Abstract
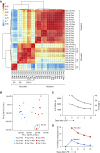
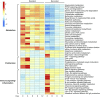
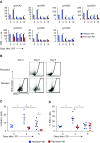
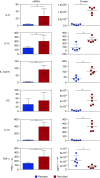
Two populations of alveolar macrophages (AMs) coexist in the inflamed lung: resident AMs that arise during embryogenesis, and recruited AMs that originate postnatally from circulating monocytes. The objective of this study was to determine whether origin or environment dictates the transcriptional, metabolic, and functional programming of these two ontologically distinct populations over the time course of acute inflammation. RNA sequencing demonstrated marked transcriptional differences between resident and recruited AMs affecting three main areas: proliferation, inflammatory signaling, and metabolism. Functional assays and metabolomic studies confirmed these differences and demonstrated that resident AMs proliferate locally and are governed by increased tricarboxylic acid cycle and amino acid metabolism. Conversely, recruited AMs produce inflammatory cytokines in association with increased glycolytic and arginine metabolism. Collectively, the data show that even though they coexist in the same environment, inflammatory macrophage subsets have distinct immunometabolic programs and perform specialized functions during inflammation that are associated with their cellular origin.
Keywords: acute lung injury; macrophage metabolism; macrophage programming.
Figures



References
-
- Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, Lohmeyer J, Herold S. Macrophage tumor necrosis factor-alpha induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med. 2009;180:521–532. - PubMed
-
- Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med. 2011;183:1380–1390. - PubMed
-
- Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, Lohmeyer J. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol. 2003;170:3273–3278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

