Synaptic input sequence discrimination on behavioral timescales mediated by reaction-diffusion chemistry in dendrites
- PMID: 28422010
- PMCID: PMC5426902
- DOI: 10.7554/eLife.25827
Synaptic input sequence discrimination on behavioral timescales mediated by reaction-diffusion chemistry in dendrites
Abstract
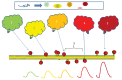
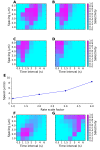
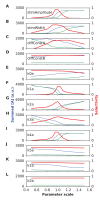
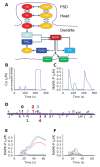
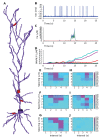
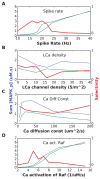
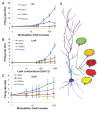
Sequences of events are ubiquitous in sensory, motor, and cognitive function. Key computational operations, including pattern recognition, event prediction, and plasticity, involve neural discrimination of spatio-temporal sequences. Here, we show that synaptically-driven reaction-diffusion pathways on dendrites can perform sequence discrimination on behaviorally relevant time-scales. We used abstract signaling models to show that selectivity arises when inputs at successive locations are aligned with, and amplified by, propagating chemical waves triggered by previous inputs. We incorporated biological detail using sequential synaptic input onto spines in morphologically, electrically, and chemically detailed pyramidal neuronal models based on rat data. Again, sequences were recognized, and local channel modulation downstream of putative sequence-triggered signaling could elicit changes in neuronal firing. We predict that dendritic sequence-recognition zones occupy 5 to 30 microns and recognize time-intervals of 0.2 to 5 s. We suggest that this mechanism provides highly parallel and selective neural computation in a functionally important time range.
Keywords: CICR; MAPK; multiscale model; neuroscience; pattern recognition; rat; reaction-diffusion; signaling.
Conflict of interest statement
USB: Reviewing editor,
Figures



Similar articles
-
Integration of synchronous synaptic input in CA1 pyramidal neuron depends on spatial and temporal distributions of the input.Hippocampus. 2013 Jan;23(1):87-99. doi: 10.1002/hipo.22061. Epub 2012 Sep 21. Hippocampus. 2013. PMID: 22996230
-
Modulation of synaptic plasticity by the coactivation of spatially distinct synaptic inputs in rat hippocampal CA1 apical dendrites.Brain Res. 2013 Aug 14;1526:1-14. doi: 10.1016/j.brainres.2013.05.023. Epub 2013 May 24. Brain Res. 2013. PMID: 23711890
-
Computational simulation of the input-output relationship in hippocampal pyramidal cells.J Comput Neurosci. 2006 Oct;21(2):191-209. doi: 10.1007/s10827-006-8797-z. Epub 2006 Jul 25. J Comput Neurosci. 2006. PMID: 16871350
-
Dendrites, deep learning, and sequences in the hippocampus.Hippocampus. 2019 Mar;29(3):239-251. doi: 10.1002/hipo.22806. Epub 2017 Oct 30. Hippocampus. 2019. PMID: 29024221 Review.
-
Influence of dendritic conductances on the input-output properties of neurons.Annu Rev Neurosci. 2001;24:653-75. doi: 10.1146/annurev.neuro.24.1.653. Annu Rev Neurosci. 2001. PMID: 11520915 Review.
Cited by
-
Discriminating neural ensemble patterns through dendritic computations in randomly connected feedforward networks.Elife. 2025 Jan 24;13:RP100664. doi: 10.7554/eLife.100664. Elife. 2025. PMID: 39854248 Free PMC article.
-
HillTau: A fast, compact abstraction for model reduction in biochemical signaling networks.PLoS Comput Biol. 2021 Nov 29;17(11):e1009621. doi: 10.1371/journal.pcbi.1009621. eCollection 2021 Nov. PLoS Comput Biol. 2021. PMID: 34843454 Free PMC article.
-
The Role of Hierarchical Dynamical Functions in Coding for Episodic Memory and Cognition.J Cogn Neurosci. 2019 Sep;31(9):1271-1289. doi: 10.1162/jocn_a_01439. Epub 2019 Jun 28. J Cogn Neurosci. 2019. PMID: 31251890 Free PMC article.
-
From membrane receptors to protein synthesis and actin cytoskeleton: Mechanisms underlying long lasting forms of synaptic plasticity.Semin Cell Dev Biol. 2019 Nov;95:120-129. doi: 10.1016/j.semcdb.2019.01.006. Epub 2019 Jan 12. Semin Cell Dev Biol. 2019. PMID: 30634048 Free PMC article. Review.
-
Geometric principles of second messenger dynamics in dendritic spines.Sci Rep. 2019 Aug 12;9(1):11676. doi: 10.1038/s41598-019-48028-0. Sci Rep. 2019. PMID: 31406140 Free PMC article.
References
-
- Abeles M. Local Cortical Circuits: An Electrophysiological Study. Berlin: Springer; 1982.
-
- Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: a central resource for neuronal morphologies. Journal of Neuroscience. 2007;27:9247–9251. doi: 10.1523/JNEUROSCI.2055-07.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

