Clarifying the Ghrelin System's Ability to Regulate Feeding Behaviours Despite Enigmatic Spatial Separation of the GHSR and Its Endogenous Ligand
- PMID: 28422060
- PMCID: PMC5412441
- DOI: 10.3390/ijms18040859
Clarifying the Ghrelin System's Ability to Regulate Feeding Behaviours Despite Enigmatic Spatial Separation of the GHSR and Its Endogenous Ligand
Abstract
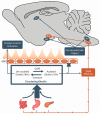
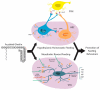
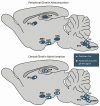
Ghrelin is a hormone predominantly produced in and secreted from the stomach. Ghrelin is involved in many physiological processes including feeding, the stress response, and in modulating learning, memory and motivational processes. Ghrelin does this by binding to its receptor, the growth hormone secretagogue receptor (GHSR), a receptor found in relatively high concentrations in hypothalamic and mesolimbic brain regions. While the feeding and metabolic effects of ghrelin can be explained by the effects of this hormone on regions of the brain that have a more permeable blood brain barrier (BBB), ghrelin produced within the periphery demonstrates a limited ability to reach extrahypothalamic regions where GHSRs are expressed. Therefore, one of the most pressing unanswered questions plaguing ghrelin research is how GHSRs, distributed in brain regions protected by the BBB, are activated despite ghrelin's predominant peripheral production and poor ability to transverse the BBB. This manuscript will describe how peripheral ghrelin activates central GHSRs to encourage feeding, and how central ghrelin synthesis and ghrelin independent activation of GHSRs may also contribute to the modulation of feeding behaviours.
Keywords: GHSR; GHSR constitutive activity; GHSR heterodimerization; blood brain barrier; central ghrelin synthesis; circumventricular organs; feeding; ghrelin; vagal afferents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Abizaid A., Liu Z.W., Andrews Z.B., Shanabrough M., Borok E., Elsworth J.D., Roth R.H., Sleeman M.W., Picciotto M.R., Tschöp M.H., et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Investig. 2006;116:3229–3239. doi: 10.1172/JCI29867. - DOI - PMC - PubMed
-
- Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S.A., Anderson J.G., Jung S., Birnbaum S., Yanagisawa M., Elmquist J.K., Nestler E.J., et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

