A comparative analysis of human and mouse islet G-protein coupled receptor expression
- PMID: 28422162
- PMCID: PMC5395952
- DOI: 10.1038/srep46600
A comparative analysis of human and mouse islet G-protein coupled receptor expression
Abstract
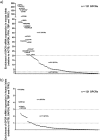
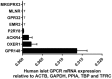
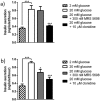
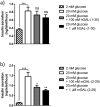
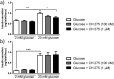
G-protein coupled receptors (GPCRs) are essential for islet function, but most studies use rodent islets due to limited human islet availability. We have systematically compared the GPCR mRNA expression in human and mouse islets to determine to what extent mouse islets can be used as surrogates for human islets to study islet GPCR function, and we have identified species-specific expression of several GPCRs. The A3 receptor (ADORA3) was expressed only in mouse islets and the A3 agonist MRS 5698 inhibited glucose-induced insulin secretion from mouse islets, with no effect on human islets. Similarly, mRNAs encoding the galanin receptors GAL1 (GALR1), GAL2 (GALR2) and GAL3 GALR3) were abundantly expressed in mouse islets but present only at low levels in human islets, so that it reads (GALR3) and galanin inhibited insulin secretion only from mouse islets. Conversely, the sst1 receptor (SSTR1) was abundant only in human islets and its selective activation by CH 275 inhibited insulin secretion from human islets, with no effect on mouse islets. Our comprehensive human and mouse islet GPCR atlas has demonstrated that species differences do exist in islet GPCR expression and function, which are likely to impact on the translatability of mouse studies to the human context.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans.Pharmacol Ther. 2013 Sep;139(3):359-91. doi: 10.1016/j.pharmthera.2013.05.004. Epub 2013 May 18. Pharmacol Ther. 2013. PMID: 23694765 Review.
-
Defining G protein-coupled receptor peptide ligand expressomes and signalomes in human and mouse islets.Cell Mol Life Sci. 2018 Aug;75(16):3039-3050. doi: 10.1007/s00018-018-2778-z. Epub 2018 Feb 17. Cell Mol Life Sci. 2018. PMID: 29455414 Free PMC article.
-
Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy.Endocrinology. 1993 Feb;132(2):879-87. doi: 10.1210/endo.132.2.8425500. Endocrinology. 1993. PMID: 8425500
-
Quantification of changes in human islet G protein-coupled receptor mRNA expression in obesity.Diabet Med. 2022 Dec;39(12):e14974. doi: 10.1111/dme.14974. Epub 2022 Nov 1. Diabet Med. 2022. PMID: 36260369
-
Inhibitory effect of kisspeptins on insulin secretion from isolated mouse islets.Diabetes Obes Metab. 2009 Nov;11 Suppl 4:197-201. doi: 10.1111/j.1463-1326.2009.01116.x. Diabetes Obes Metab. 2009. PMID: 19817802 Review.
Cited by
-
Cryo-electron microscopy for GPCR research and drug discovery in endocrinology and metabolism.Nat Rev Endocrinol. 2024 Jun;20(6):349-365. doi: 10.1038/s41574-024-00957-1. Epub 2024 Feb 29. Nat Rev Endocrinol. 2024. PMID: 38424377 Review.
-
Sex Differences in Androgen Regulation of Metabolism in Nonhuman Primates.Adv Exp Med Biol. 2017;1043:559-574. doi: 10.1007/978-3-319-70178-3_24. Adv Exp Med Biol. 2017. PMID: 29224110 Free PMC article. Review.
-
Targeting lipid GPCRs to treat type 2 diabetes mellitus - progress and challenges.Nat Rev Endocrinol. 2021 Mar;17(3):162-175. doi: 10.1038/s41574-020-00459-w. Epub 2021 Jan 25. Nat Rev Endocrinol. 2021. PMID: 33495605 Review.
-
The effects of liraglutide on expressions of insulin secretion and beta cell survive associated GPCR genes in pancreatic beta cells.Turk J Med Sci. 2025 Mar 20;55(2):525-530. doi: 10.55730/1300-0144.5997. eCollection 2025. Turk J Med Sci. 2025. PMID: 40342327 Free PMC article.
-
RFX6 Maintains Gene Expression and Function of Adult Human Islet α-Cells.Diabetes. 2024 Mar 1;73(3):448-460. doi: 10.2337/db23-0483. Diabetes. 2024. PMID: 38064570 Free PMC article.
References
-
- Amisten S., Salehi A., Rorsman P., Jones P. M. & Persaud S. J. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacology & therapeutics 139, 359–391 (2013). - PubMed
-
- Ahren B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia 43, 393–410 (2000). - PubMed
-
- Jones P. M. P. S. Textbook of Diabetes. In: Textbook of Diabetes (ed^(eds Holt G, Flyvberg). Blackwell Scientific Press, UK (2010).
-
- Tuch B. E. Clinical use of GLP-1 agonists and DPP4 inhibitors. Pancreatology 16, 8–9 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

