Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine
- PMID: 28422178
- PMCID: PMC5395940
- DOI: 10.1038/srep46621
Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine
Abstract
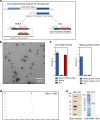
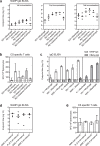
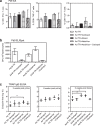
The leading malaria vaccine in development is the circumsporozoite protein (CSP)-based particle vaccine, RTS,S, which targets the pre-erythrocytic stage of Plasmodium falciparum infection. It induces modest levels of protective efficacy, thought to be mediated primarily by CSP-specific antibodies. We aimed to enhance vaccine efficacy by generating a more immunogenic CSP-based particle vaccine and therefore developed a next-generation RTS,S-like vaccine, called R21. The major improvement is that in contrast to RTS,S, R21 particles are formed from a single CSP-hepatitis B surface antigen (HBsAg) fusion protein, and this leads to a vaccine composed of a much higher proportion of CSP than in RTS,S. We demonstrate that in BALB/c mice R21 is immunogenic at very low doses and when administered with the adjuvants Abisco-100 and Matrix-M it elicits sterile protection against transgenic sporozoite challenge. Concurrent induction of potent cellular and humoral immune responses was also achieved by combining R21 with TRAP-based viral vectors and protective efficacy was significantly enhanced. In addition, in contrast to RTS,S, only a minimal antibody response to the HBsAg carrier was induced. These studies identify an anti-sporozoite vaccine component that may improve upon the current leading malaria vaccine RTS,S. R21 is now under evaluation in Phase 1/2a clinical trials.
Conflict of interest statement
Katharine A. Collins, Sarah C. Gilbert, Adrian V. S. Hill are named as co-inventors on a patent filing related to the R21 immunogen. M.G.C. and R.S declare no competing financial interests.
Figures



References
-
- WHO. World Malaria Report 2015. World Health Organisation (2015).
-
- Mendis K. et al. From malaria control to eradication: The WHO perspective. Trop Med Int Health 14, 802–809 (2009). - PubMed
-
- Hoffman S. L. et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 185, 1155–1164 (2002). - PubMed
-
- Roestenberg M. et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361, 468–477 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

