Fbxw7 haploinsufficiency loses its protection against DNA damage and accelerates MNU-induced gastric carcinogenesis
- PMID: 28422719
- PMCID: PMC5464881
- DOI: 10.18632/oncotarget.16800
Fbxw7 haploinsufficiency loses its protection against DNA damage and accelerates MNU-induced gastric carcinogenesis
Abstract
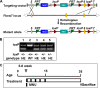
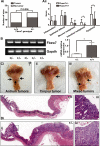
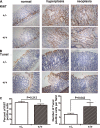
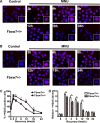
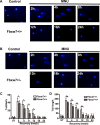
Fbxw7, a subunit of the SCF E3 ubiquitin ligase, recognizes oncoprotein substrates and leads to their proteasomal degradation. Fbxw7 acts as a tumor suppressor via inducing apoptosis and growth arrest in various kinds of tumors. To clarify the initiating role in gastric carcinogenesis as well as the histologic characterization of tumor in Fbxw7 allele haploinsufficient mice, we generated Fbxw7 heterozygous knockout mice (Fbxw7+/-) and treated them with chemical carcinogen N-methyl-N-nitrosourea (MNU) at 5-6 weeks of age. We also treated mouse embryo fibroblasts (MEFs) from Fbxw7+/- and Fbxw7+/+ mice with MNU and examined cell DNA damage via comet assay. The protein expression of Fbxw7 and its substrate c-Myc from mouse tumors, as well as human tumors sampled from six patients, were detected by Western blot. As results, the tumor incidence was obviously higher in Fbxw7+/- mice (13/20) than in Fbxw7+/+ mice (6/20) after 35-week observation. Intestinal metaplasia (P = 0.013) and dysplasia (P = 0.036) were more severe in Fbxw7+/- mice than in Fbxw7+/+ mice. The repair potential of DNA damage was suppressed in MEFs from Fbxw7+/- mice after MNU exposure. Increased c-Myc expression was accompanied by decreased Fbxw7 protein expression in tumor tissues from mouse and patients. In conclusion, Fbxw7 haploinsufficiency increased the risk of gastric carcinogenesis induced by MNU, which is associated with the accumulation of DNA damage as well as c-Myc oncoprotein.
Keywords: DNA damage; Fbxw7; N-Methyl-N-nitrosourea; gastric cancer; knockout mouse.
Conflict of interest statement
None.
Figures

References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer Journal international du cancer. 2015;136:E359–386. - PubMed
-
- Tan Y, Sangfelt O, Spruck C. The Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer letters. 2008;271:1–12. - PubMed
-
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nature reviews Cancer. 2008;8:83–93. - PubMed
-
- Cheng Y, Li G. Role of the ubiquitin ligase Fbw7 in cancer progression. Cancer metastasis reviews. 2012;31:75–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

