Calmodulin promotes matrix metalloproteinase 9 production and cell migration by inhibiting the ubiquitination and degradation of TBC1D3 oncoprotein in human breast cancer cells
- PMID: 28422741
- PMCID: PMC5482662
- DOI: 10.18632/oncotarget.16756
Calmodulin promotes matrix metalloproteinase 9 production and cell migration by inhibiting the ubiquitination and degradation of TBC1D3 oncoprotein in human breast cancer cells
Abstract
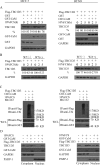
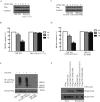
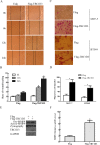
The hominoid oncoprotein TBC1D3 enhances growth factor (GF) signaling and GF signaling, conversely, induces the ubiquitination and subsequent degradation of TBC1D3. However, little is known regarding the regulation of this degradation, and the role of TBC1D3 in the progression of tumors has also not been defined. In the present study, we demonstrated that calmodulin (CaM), a ubiquitous cellular calcium sensor, specifically interacted with TBC1D3 in a Ca2+-dependent manner and inhibited GF signaling-induced ubiquitination and degradation of the oncoprotein in both cytoplasm and nucleus of human breast cancer cells. The CaM-interacting site of TBC1D3 was mapped to amino acids 157~171, which comprises two 1-14 hydrophobic motifs and one lysine residue (K166). Deletion of these motifs was shown to abolish interaction between TBC1D3 and CaM. Surprisingly, this deletion mutation caused inability of GF signaling to induce the ubiquitination and subsequent degradation of TBC1D3. In agreement with this, we identified lysine residue 166 within the CaM-interacting motifs of TBC1D3 as the actual site for the GF signaling-induced ubiquitination using mutational analysis. Point mutation of this lysine residue exhibited the same effect on TBC1D3 as the deletion mutant, suggesting that CaM inhibits GF signaling-induced degradation of TBC1D3 by occluding its ubiquitination at K166. Notably, we found that TBC1D3 promoted the expression and activation of MMP-9 and the migration of MCF-7 cells. Furthermore, interaction with CaM considerably enhanced such effect of TBC1D3. Taken together, our work reveals a novel model by which CaM promotes cell migration through inhibiting the ubiquitination and degradation of TBC1D3.
Keywords: TBC1D3; calmodulin; cell migration; protein degradation; protein ubiquitination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

