Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism
- PMID: 28422753
- PMCID: PMC5396526
- DOI: 10.1172/jci.insight.93136
Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism
Abstract
Background: Adrenal aldosterone excess is the most common cause of secondary hypertension and is associated with increased cardiovascular morbidity. However, adverse metabolic risk in primary aldosteronism extends beyond hypertension, with increased rates of insulin resistance, type 2 diabetes, and osteoporosis, which cannot be easily explained by aldosterone excess.
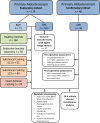
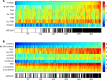
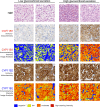
Methods: We performed mass spectrometry-based analysis of a 24-hour urine steroid metabolome in 174 newly diagnosed patients with primary aldosteronism (103 unilateral adenomas, 71 bilateral adrenal hyperplasias) in comparison to 162 healthy controls, 56 patients with endocrine inactive adrenal adenoma, 104 patients with mild subclinical, and 47 with clinically overt adrenal cortisol excess. We also analyzed the expression of cortisol-producing CYP11B1 and aldosterone-producing CYP11B2 enzymes in adenoma tissue from 57 patients with aldosterone-producing adenoma, employing immunohistochemistry with digital image analysis.
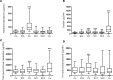
Results: Primary aldosteronism patients had significantly increased cortisol and total glucocorticoid metabolite excretion (all P < 0.001), only exceeded by glucocorticoid output in patients with clinically overt adrenal Cushing syndrome. Several surrogate parameters of metabolic risk correlated significantly with glucocorticoid but not mineralocorticoid output. Intratumoral CYP11B1 expression was significantly associated with the corresponding in vivo glucocorticoid excretion. Unilateral adrenalectomy resolved both mineralocorticoid and glucocorticoid excess. Postoperative evidence of adrenal insufficiency was found in 13 (29%) of 45 consecutively tested patients.
Conclusion: Our data indicate that glucocorticoid cosecretion is frequently found in primary aldosteronism and contributes to associated metabolic risk. Mineralocorticoid receptor antagonist therapy alone may not be sufficient to counteract adverse metabolic risk in medically treated patients with primary aldosteronism.
Funding: Medical Research Council UK, Wellcome Trust, European Commission.
Keywords: Cardiology; Endocrinology.
Conflict of interest statement
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

