Evaluation of gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma
- PMID: 28422841
- PMCID: PMC5406057
- DOI: 10.1097/MD.0000000000006544
Evaluation of gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma
Abstract
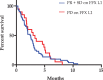
To evaluate gemcitabine efficacy in advanced pancreatic cancer patients after the FOLFIRINOX regimen.Patients with locally-advanced or metastatic pancreatic adenocarcinoma from French and Canadian centers, who were treated with the first-line FOLFIRINOX regimen (FFX L1), followed by gemcitabine monotherapy as a second-line treatment (GEM L2), were retrospectively evaluated. Statistical analyses were performed on the demographic, toxicity, and response rate data. Overall survival (OS) and progression-free survival (PFS) were assessed using the Kaplan-Meier method.Seventy-two patients were reviewed (median age of 63.5 years [range, 32-75 years], men [62%], predominantly pancreatic head tumor location [51%] and metastatic disease [64%] at the time of diagnosis). The objective response rate to GEM-L2 treatment was 8/72 (11%), and 32 patients (44%) experienced a clinical benefit from gemcitabine. Four patients had a partial response to GEM-L2, although they previously showed a progressive response following FFX-L1 treatment. The median OS for the entire cohort was 13.6 months (95% confidence interval [CI]: 2.0-35). The median PFS of the GEM-L2 group was 2.5 months (95% CI: 0.2-10.8) with no statistical differences between patients with controlled or progressive disease on FFX-L1 therapy.Gemcitabine as a second-line treatment for advanced pancreatic adenocarcinoma after FOLFIRINOX failure showed clinical benefits in some patients.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. - PubMed
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics 2010. CA Cancer J Clin 2010;60:277–300. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. - PubMed
-
- Calvo F, Guillen Ponce C, Muñoz Beltran M, et al. Multidisciplinary management of locally advanced-borderline resectable adenocarcinoma of the head of the pancreas. Clin Transl Oncol 2013;15:173–81. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials