Towards a theory of cortical columns: From spiking neurons to interacting neural populations of finite size
- PMID: 28422957
- PMCID: PMC5415267
- DOI: 10.1371/journal.pcbi.1005507
Towards a theory of cortical columns: From spiking neurons to interacting neural populations of finite size
Abstract
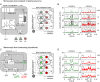
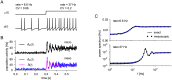
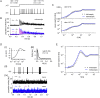
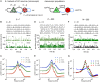
Neural population equations such as neural mass or field models are widely used to study brain activity on a large scale. However, the relation of these models to the properties of single neurons is unclear. Here we derive an equation for several interacting populations at the mesoscopic scale starting from a microscopic model of randomly connected generalized integrate-and-fire neuron models. Each population consists of 50-2000 neurons of the same type but different populations account for different neuron types. The stochastic population equations that we find reveal how spike-history effects in single-neuron dynamics such as refractoriness and adaptation interact with finite-size fluctuations on the population level. Efficient integration of the stochastic mesoscopic equations reproduces the statistical behavior of the population activities obtained from microscopic simulations of a full spiking neural network model. The theory describes nonlinear emergent dynamics such as finite-size-induced stochastic transitions in multistable networks and synchronization in balanced networks of excitatory and inhibitory neurons. The mesoscopic equations are employed to rapidly integrate a model of a cortical microcircuit consisting of eight neuron types, which allows us to predict spontaneous population activities as well as evoked responses to thalamic input. Our theory establishes a general framework for modeling finite-size neural population dynamics based on single cell and synapse parameters and offers an efficient approach to analyzing cortical circuits and computations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Mind the last spike - firing rate models for mesoscopic populations of spiking neurons.Curr Opin Neurobiol. 2019 Oct;58:155-166. doi: 10.1016/j.conb.2019.08.003. Epub 2019 Oct 4. Curr Opin Neurobiol. 2019. PMID: 31590003 Review.
-
A stochastic-field description of finite-size spiking neural networks.PLoS Comput Biol. 2017 Aug 7;13(8):e1005691. doi: 10.1371/journal.pcbi.1005691. eCollection 2017 Aug. PLoS Comput Biol. 2017. PMID: 28787447 Free PMC article.
-
Low-dimensional spike rate models derived from networks of adaptive integrate-and-fire neurons: Comparison and implementation.PLoS Comput Biol. 2017 Jun 23;13(6):e1005545. doi: 10.1371/journal.pcbi.1005545. eCollection 2017 Jun. PLoS Comput Biol. 2017. PMID: 28644841 Free PMC article.
-
A master equation formalism for macroscopic modeling of asynchronous irregular activity states.Neural Comput. 2009 Jan;21(1):46-100. doi: 10.1162/neco.2009.02-08-710. Neural Comput. 2009. PMID: 19210171
-
A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input.Biol Cybern. 2006 Jul;95(1):1-19. doi: 10.1007/s00422-006-0068-6. Epub 2006 Apr 19. Biol Cybern. 2006. PMID: 16622699 Review.
Cited by
-
Columnar connectome: toward a mathematics of brain function.Netw Neurosci. 2019 Jul 1;3(3):779-791. doi: 10.1162/netn_a_00088. eCollection 2019. Netw Neurosci. 2019. PMID: 31410379 Free PMC article.
-
Winnerless competition in clustered balanced networks: inhibitory assemblies do the trick.Biol Cybern. 2018 Apr;112(1-2):81-98. doi: 10.1007/s00422-017-0737-7. Epub 2017 Oct 26. Biol Cybern. 2018. PMID: 29075845 Free PMC article.
-
Cortical state dynamics and selective attention define the spatial pattern of correlated variability in neocortex.Nat Commun. 2022 Jan 10;13(1):44. doi: 10.1038/s41467-021-27724-4. Nat Commun. 2022. PMID: 35013259 Free PMC article.
-
Mesoscopic description of hippocampal replay and metastability in spiking neural networks with short-term plasticity.PLoS Comput Biol. 2022 Dec 22;18(12):e1010809. doi: 10.1371/journal.pcbi.1010809. eCollection 2022 Dec. PLoS Comput Biol. 2022. PMID: 36548392 Free PMC article.
-
Bifurcation analysis of the dynamics of interacting subnetworks of a spiking network.Sci Rep. 2019 Aug 6;9(1):11397. doi: 10.1038/s41598-019-47190-9. Sci Rep. 2019. PMID: 31388027 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

