Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain
- PMID: 28422959
- PMCID: PMC5396861
- DOI: 10.1371/journal.pbio.2001573
Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain
Abstract
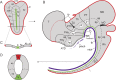
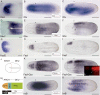
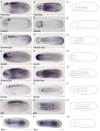
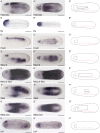
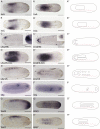
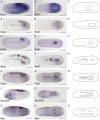
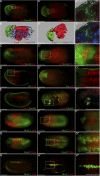
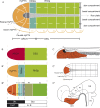
All vertebrate brains develop following a common Bauplan defined by anteroposterior (AP) and dorsoventral (DV) subdivisions, characterized by largely conserved differential expression of gene markers. However, it is still unclear how this Bauplan originated during evolution. We studied the relative expression of 48 genes with key roles in vertebrate neural patterning in a representative amphioxus embryonic stage. Unlike nonchordates, amphioxus develops its central nervous system (CNS) from a neural plate that is homologous to that of vertebrates, allowing direct topological comparisons. The resulting genoarchitectonic model revealed that the amphioxus incipient neural tube is unexpectedly complex, consisting of several AP and DV molecular partitions. Strikingly, comparison with vertebrates indicates that the vertebrate thalamus, pretectum, and midbrain domains jointly correspond to a single amphioxus region, which we termed Di-Mesencephalic primordium (DiMes). This suggests that these domains have a common developmental and evolutionary origin, as supported by functional experiments manipulating secondary organizers in zebrafish and mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Nieuwenhuys R, Puelles L. Towards a New Neuromorphology. Cham: Springer International Publishing; 2016.
-
- Puelles L, Martinez-de-la-Torre M, Bardet SM, Rubenstein JLR. Hypothalamus In: Watson C, Paxinos G, Puelles L, editors. The mouse neurvous system, London, San Diego, CA: Academic Press/Elsevier; 2012, p. 221–312.
-
- Puelles L. Plan of the Developing Vertebrate Nervous System In: Rubenstein JLR, Rakic P, editors. Pattering and Cell Type Specification in the Developing CNS and PNS, vol. 1, Amsterdam: Academic Press; 2013, p. 187–209.
-
- Puelles L, Rubenstein JLR. A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model. Front Neuroanat 2015;9:27 doi: 10.3389/fnana.2015.00027 - DOI - PMC - PubMed
-
- Hidalgo-Sánchez M, Millet S, Bloch-Gallego E, Alvarado-Mallart RM. Specification of the meso-isthmo-cerebellar region: The Otx2/Gbx2 boundary. Brain Res Rev 2005;49:134–49. doi: 10.1016/j.brainresrev.2005.01.010 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

