The gut microbiota as a modulator of innate immunity during melioidosis
- PMID: 28422970
- PMCID: PMC5411098
- DOI: 10.1371/journal.pntd.0005548
The gut microbiota as a modulator of innate immunity during melioidosis
Abstract
Background: Melioidosis, caused by the Gram-negative bacterium Burkholderia pseudomallei, is an emerging cause of pneumonia-derived sepsis in the tropics. The gut microbiota supports local mucosal immunity and is increasingly recognized as a protective mediator in host defenses against systemic infection. Here, we aimed to characterize the composition and function of the intestinal microbiota during experimental melioidosis.
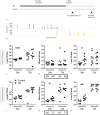
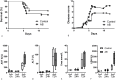
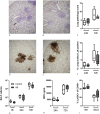
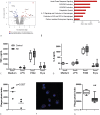
Methodology/principal findings: C57BL/6 mice were infected intranasally with B. pseudomallei and sacrificed at different time points to assess bacterial loads and inflammation. In selected experiments, the gut microbiota was disrupted with broad-spectrum antibiotics prior to inoculation. Fecal bacterial composition was analyzed by means of IS-pro, a 16S-23S interspacer region-based profiling method. A marked shift in fecal bacterial composition was seen in all mice during systemic B. pseudomallei infection with a strong increase in Proteobacteria and decrease in Actinobacteria, with an increase in bacterial diversity. We found enhanced early dissemination of B. pseudomallei and systemic inflammation during experimental melioidosis in microbiota-disrupted mice compared with controls. Whole-genome transcriptional profiling of the lung identified several genes that were differentially expressed between mice with a normal or disrupted intestinal microbiota. Genes involved in acute phase signaling, including macrophage-related signaling pathways were significantly elevated in microbiota disrupted mice. Compared with controls, alveolar macrophages derived from antibiotic pretreated mice showed a diminished capacity to phagocytose B. pseudomallei. This might in part explain the observed protective effect of the gut microbiota in the host defense against pneumonia-derived melioidosis.
Conclusions/significance: Taken together, these data identify the gut microbiota as a potential modulator of innate immunity during B. pseudomallei infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia.Gut. 2016 Apr;65(4):575-83. doi: 10.1136/gutjnl-2015-309728. Epub 2015 Oct 28. Gut. 2016. PMID: 26511795 Free PMC article.
-
Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis).PLoS Med. 2007 Jul 31;4(7):e248. doi: 10.1371/journal.pmed.0040248. PLoS Med. 2007. PMID: 17676990 Free PMC article.
-
MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei.PLoS One. 2008;3(10):e3494. doi: 10.1371/journal.pone.0003494. Epub 2008 Oct 23. PLoS One. 2008. PMID: 18946505 Free PMC article.
-
The molecular and cellular basis of pathogenesis in melioidosis: how does Burkholderia pseudomallei cause disease?FEMS Microbiol Rev. 2009 Nov;33(6):1079-99. doi: 10.1111/j.1574-6976.2009.00189.x. Epub 2009 Aug 5. FEMS Microbiol Rev. 2009. PMID: 19732156 Review.
-
Immunity to Burkholderia pseudomallei.Curr Opin Infect Dis. 2009 Apr;22(2):102-8. doi: 10.1097/QCO.0b013e328322e727. Curr Opin Infect Dis. 2009. PMID: 19276877 Review.
Cited by
-
Long-distance relationships - regulation of systemic host defense against infections by the gut microbiota.Mucosal Immunol. 2022 May;15(5):809-818. doi: 10.1038/s41385-022-00539-2. Epub 2022 Jun 22. Mucosal Immunol. 2022. PMID: 35732817 Review.
-
Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis.Front Immunol. 2018 Sep 10;9:2042. doi: 10.3389/fimmu.2018.02042. eCollection 2018. Front Immunol. 2018. PMID: 30250472 Free PMC article. Review.
-
Plant Polysaccharides Modulate Immune Function via the Gut Microbiome and May Have Potential in COVID-19 Therapy.Molecules. 2022 Apr 26;27(9):2773. doi: 10.3390/molecules27092773. Molecules. 2022. PMID: 35566123 Free PMC article. Review.
-
Exploring the complex interplay: gut microbiome, stress, and leptospirosis.Front Microbiol. 2024 Feb 27;15:1345684. doi: 10.3389/fmicb.2024.1345684. eCollection 2024. Front Microbiol. 2024. PMID: 38476949 Free PMC article. Review.
-
Pulmonary and intestinal microbiota dynamics during Gram-negative pneumonia-derived sepsis.Intensive Care Med Exp. 2021 Jul 12;9(1):35. doi: 10.1186/s40635-021-00398-4. Intensive Care Med Exp. 2021. PMID: 34250564 Free PMC article.
References
-
- Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nature Microbiology. 2016;1:15008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

