Determination of influenza B identity and potency in quadrivalent inactivated influenza vaccines using lineage-specific monoclonal antibodies
- PMID: 28423025
- PMCID: PMC5396888
- DOI: 10.1371/journal.pone.0175733
Determination of influenza B identity and potency in quadrivalent inactivated influenza vaccines using lineage-specific monoclonal antibodies
Abstract
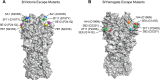
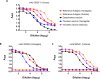
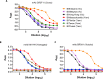
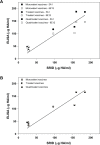
Co-circulation of two antigenically and genetically distinct lineages of influenza B virus, represented by prototype viruses B/Victoria/2/1987 and B/Yamagata/16/1988, has led to the development of quadrivalent influenza vaccines that contain two influenza B antigens. The inclusion of two influenza B antigens presents challenges for the production and regulation of inactivated quadrivalent vaccines, including the potential for cross-reactivity of the reagents used in identity and potency assays because of the relative close relatedness of the hemagglutinin (HA) from the two virus lineages. Monoclonal antibodies (mAbs) specific for the two lineages of influenza B HA were generated and characterized and used to set-up simple identity tests that distinguish the influenza B antigens in inactivated trivalent and quadrivalent vaccines. The lineage-specific mAbs bound well to the HA of influenza B strains included in influenza vaccines over a period of more than 10 years, suggesting that identity tests using such lineage-specific mAbs would not necessarily have to be updated with every influenza B vaccine strain change. These lineage-specific mAbs were also used in an antibody capture ELISA format to quantify HA in vaccine samples, including monovalent, trivalent, and quadrivalent vaccine samples from various manufacturers. The results demonstrated correlation with HA values determined by the traditional single radial immunodiffusion (SRID) assay. Further, the antibody-capture ELISA was able to distinguish heat-stressed vaccine from unstressed vaccine, and was similar to the SRID in quantifying the resultant loss of potency. These mAb reagents should be useful for further development of antibody-based alternative influenza B identity and potency assays.
Conflict of interest statement
Figures

Similar articles
-
A monoclonal antibody-based immunoassay for measuring the potency of 2009 pandemic influenza H1N1 vaccines.Influenza Other Respir Viruses. 2014 Sep;8(5):587-95. doi: 10.1111/irv.12272. Epub 2014 Aug 2. Influenza Other Respir Viruses. 2014. PMID: 25087462 Free PMC article.
-
Analysis of the vaccine-induced influenza B virus hemagglutinin-specific antibody dependent cellular cytotoxicity response.Virus Res. 2020 Feb;277:197839. doi: 10.1016/j.virusres.2019.197839. Epub 2019 Dec 16. Virus Res. 2020. PMID: 31837382 Clinical Trial.
-
Potency determination of inactivated H7 influenza vaccines using monoclonal antibody-based ELISA and biolayer interferometry assays.Influenza Other Respir Viruses. 2018 Mar;12(2):250-258. doi: 10.1111/irv.12528. Epub 2017 Dec 15. Influenza Other Respir Viruses. 2018. PMID: 29152878 Free PMC article.
-
[Immunogenicity of inacitivated quadrivalent influenza vaccine in adults aged 18-64 years: A systematic review and Meta-analysis].Zhonghua Liu Xing Bing Xue Za Zhi. 2018 Dec 10;39(12):1636-1641. doi: 10.3760/cma.j.issn.0254-6450.2018.12.019. Zhonghua Liu Xing Bing Xue Za Zhi. 2018. PMID: 30572392 Chinese.
-
Evidence update: GlaxoSmithKline's inactivated quadrivalent influenza vaccines.Expert Rev Vaccines. 2016;15(2):201-14. doi: 10.1586/14760584.2016.1113878. Epub 2015 Dec 5. Expert Rev Vaccines. 2016. PMID: 26641539 Review.
Cited by
-
Broad Reactivity Single Domain Antibodies against Influenza Virus and Their Applications to Vaccine Potency Testing and Immunotherapy.Biomolecules. 2021 Mar 10;11(3):407. doi: 10.3390/biom11030407. Biomolecules. 2021. PMID: 33802072 Free PMC article. Review.
-
Correlation of Influenza B Haemagglutination Inhibiton, Single-Radial Haemolysis and Pseudotype-Based Microneutralisation Assays for Immunogenicity Testing of Seasonal Vaccines.Vaccines (Basel). 2021 Jan 28;9(2):100. doi: 10.3390/vaccines9020100. Vaccines (Basel). 2021. PMID: 33525543 Free PMC article.
-
Rapid determination of influenza vaccine potency by an SPR-based method using subtype or lineage-specific monoclonal antibodies.Front Immunol. 2023 Jun 29;14:1128683. doi: 10.3389/fimmu.2023.1128683. eCollection 2023. Front Immunol. 2023. PMID: 37457687 Free PMC article.
-
Susceptibility of influenza viruses to hypothiocyanite and hypoiodite produced by lactoperoxidase in a cell-free system.PLoS One. 2018 Jul 25;13(7):e0199167. doi: 10.1371/journal.pone.0199167. eCollection 2018. PLoS One. 2018. PMID: 30044776 Free PMC article.
-
Development of Lentiviral Vectors Pseudotyped With Influenza B Hemagglutinins: Application in Vaccine Immunogenicity, mAb Potency, and Sero-Surveillance Studies.Front Immunol. 2021 May 24;12:661379. doi: 10.3389/fimmu.2021.661379. eCollection 2021. Front Immunol. 2021. PMID: 34108964 Free PMC article.
References
-
- Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, et al. (1990) Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 175: 59–68. - PubMed
-
- Ambrose CS and Levin MJ (2012) The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother 8: 81–88. doi: 10.4161/hv.8.1.17623 - DOI - PMC - PubMed
-
- Belshe RB (2010) The need for quadrivalent vaccine against seasonal influenza. Vaccine 28 Suppl 4: D45–53. - PubMed
-
- Weir JP and Gruber MF (2016) An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir Viruses 10: 354–360. doi: 10.1111/irv.12383 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

