Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047
- PMID: 28423064
- PMCID: PMC5396973
- DOI: 10.1371/journal.pone.0175917
Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047
Erratum in
-
Correction: Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047.PLoS One. 2017 May 16;12(5):e0178102. doi: 10.1371/journal.pone.0178102. eCollection 2017. PLoS One. 2017. PMID: 28520822 Free PMC article.
Abstract
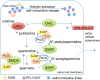
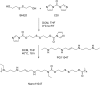
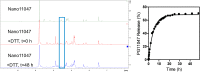
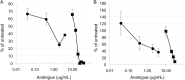
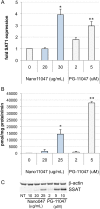
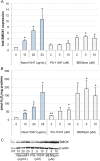
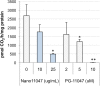
Synthesizing polycationic polymers directly from existing drugs overcomes the drug-loading limitations often associated with pharmacologically inert nanocarriers. We recently described nanocarriers formed from a first-generation polyamine analogue, bis(ethyl)norspermine (BENSpm), that could simultaneously target polyamine metabolism while delivering therapeutic nucleic acids. In the current study, we describe the synthesis and evaluation of self-immolative nanocarriers derived from the second-generation polyamine analogue PG-11047. Polyamines are absolutely essential for proliferation and their metabolism is frequently dysregulated in cancer. Through its effects on polyamine metabolism, PG-11047 effectively inhibits tumor growth in cancer cell lines of multiple origins as well as in human tumor mouse xenografts. Promising clinical trials have been completed verifying the safety and tolerance of this rotationally restricted polyamine analogue. We therefore used PG-11047 as the basis for Nano11047, a biodegradable, prodrug nanocarrier capable of targeting polyamine metabolism. Following exposure of lung cancer cell lines to Nano11047, uptake and intracellular degradation into the parent compound PG-11047 was observed. The release of PG-11047 highly induced the polyamine catabolic enzyme activities of spermidine/spermine N1-acetyltransferase (SSAT) and spermine oxidase (SMOX). By contrast, the activity of ornithine decarboxylase (ODC), a rate-limiting enzyme in polyamine biosynthesis and a putative oncogene, was decreased. Consequently, intracellular levels of the natural polyamines were depleted concurrent with tumor cell growth inhibition. This availability of Nano11047 as a novel drug form and potential nucleic acid delivery vector will potentially benefit and encourage future clinical studies.
Conflict of interest statement
Figures
References
-
- Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58(2): 244–258. doi: 10.1007/PL00000852 - DOI - PMC - PubMed
-
- Balasundaram D, Tyagi AK. Polyamine—DNA nexus: structural ramifications and biological implications. Mol Cell Biochem. 1991;100(2): 129–140. - PubMed
-
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42(1): 39–51. doi: 10.1016/j.biocel.2009.07.009 - DOI - PubMed
-
- Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9(1): 1–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

