Functional identification of activity-regulated, high-affinity glutamine transport in hippocampal neurons inhibited by riluzole
- PMID: 28423185
- PMCID: PMC5594568
- DOI: 10.1111/jnc.14046
Functional identification of activity-regulated, high-affinity glutamine transport in hippocampal neurons inhibited by riluzole
Abstract
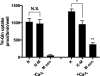
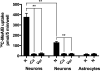
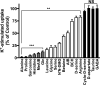
Glutamine (Gln) is considered the preferred precursor for the neurotransmitter pool of glutamate (Glu), the major excitatory transmitter in the mammalian CNS. Here, an activity-regulated, high-affinity Gln transport system is described in developing and mature neuron-enriched hippocampal cultures that is potently inhibited by riluzole (IC50 1.3 ± 0.5 μM), an anti-glutamatergic drug, and is blocked by low concentrations of 2-(methylamino)isobutyrate (MeAIB), a system A transport inhibitor. K+ -stimulated MeAIB transport displays an affinity (Km ) for MeAIB of 37 ± 1.2 μM, saturates at ~ 200 μM, is dependent on extracellular Ca2+ , and is blocked by inhibition of voltage-gated Ca2+ channels. Spontaneous MeAIB transport is also dependent on extracellullar Ca2+ and voltage-gated calcium channels, but is also blocked by the Na+ channel blocker tetrodotoxin, by Glu receptor antagonists, and by GABA indicating its dependence on intact neural circuits driven by endogenous glutamatergic activity. The transport of MeAIB itself does not rely on Ca2+ , but on Na+ ions, and is pH sensitive. Activity-regulated, riluzole-sensitive spontaneous and K+ -stimulated transport is minimal at 7-8 days in vitro, coordinately induced during the next 2 weeks and is maximally expressed by days in vitro > 20; the known period for maturation of the Glu/Gln cycle and regulated pre-synaptic Glu release. Competition analyses with various amino acids indicate that Gln is the most likely physiological substrate. Activity-regulated Gln/MeAIB transport is not observed in astrocytes. The functional identification of activity-regulated, high-affinity, riluzole-sensitive Gln/MeAIB transport in hippocampal neurons may have important ramifications in the neurobiology of activity-stimulated pre-synaptic Glu release, the Glu/Gln cycle between astrocytes and neurons, and neuronal Glu-induced excitotoxicity. Cover Image for this issue: doi: 10.1111/jnc.13805.
Keywords: activity-dependent regulation; excitotoxicity; glutamate/glutamine cycle; neuronal glutamine transporter; neuroprotection; neurotransmitter cycling.
© 2017 International Society for Neurochemistry.
Conflict of interest statement
Figures
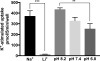



Similar articles
-
Ca2+-regulated expression of high affinity methylaminoisobutryic acid transport in hippocampal neurons inhibited by riluzole and novel neuroprotective aminothiazoles.Curr Res Physiol. 2023 Oct 14;6:100109. doi: 10.1016/j.crphys.2023.100109. eCollection 2023. Curr Res Physiol. 2023. PMID: 38107787 Free PMC article.
-
Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1.J Neurosci. 2006 Aug 16;26(33):8537-48. doi: 10.1523/JNEUROSCI.0329-06.2006. J Neurosci. 2006. PMID: 16914680 Free PMC article.
-
The protective effects of riluzole on manganese-induced disruption of glutamate transporters and glutamine synthetase in the cultured astrocytes.Biol Trace Elem Res. 2012 Aug;148(2):242-9. doi: 10.1007/s12011-012-9365-1. Epub 2012 Mar 6. Biol Trace Elem Res. 2012. PMID: 22391793
-
Roles of glutamine in neurotransmission.Neuron Glia Biol. 2010 Nov;6(4):263-76. doi: 10.1017/S1740925X11000093. Epub 2011 Oct 21. Neuron Glia Biol. 2010. PMID: 22018046 Review.
-
Roles of Glutamate and Glutamine Transport in Ammonia Neurotoxicity: State of the Art and Question Marks.Endocr Metab Immune Disord Drug Targets. 2018;18(4):306-315. doi: 10.2174/1871520618666171219124427. Endocr Metab Immune Disord Drug Targets. 2018. PMID: 29256360 Review.
Cited by
-
Riluzole and novel naphthalenyl substituted aminothiazole derivatives prevent acute neural excitotoxic injury in a rat model of temporal lobe epilepsy.Neuropharmacology. 2023 Feb 15;224:109349. doi: 10.1016/j.neuropharm.2022.109349. Epub 2022 Nov 24. Neuropharmacology. 2023. PMID: 36436594 Free PMC article.
-
D-Serine Signaling and NMDAR-Mediated Synaptic Plasticity Are Regulated by System A-Type of Glutamine/D-Serine Dual Transporters.J Neurosci. 2020 Aug 19;40(34):6489-6502. doi: 10.1523/JNEUROSCI.0801-20.2020. Epub 2020 Jul 13. J Neurosci. 2020. PMID: 32661027 Free PMC article.
-
Targeting NMDA Receptor Complex in Management of Epilepsy.Pharmaceuticals (Basel). 2022 Oct 21;15(10):1297. doi: 10.3390/ph15101297. Pharmaceuticals (Basel). 2022. PMID: 36297409 Free PMC article. Review.
-
Neurotransmitters in Prevention and Treatment of Alzheimer's Disease.Int J Mol Sci. 2023 Feb 14;24(4):3841. doi: 10.3390/ijms24043841. Int J Mol Sci. 2023. PMID: 36835251 Free PMC article. Review.
-
Glutamate Transporters and Mitochondria: Signaling, Co-compartmentalization, Functional Coupling, and Future Directions.Neurochem Res. 2020 Mar;45(3):526-540. doi: 10.1007/s11064-020-02974-8. Epub 2020 Jan 30. Neurochem Res. 2020. PMID: 32002773 Free PMC article. Review.
References
-
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–343. - PubMed
-
- Armano S, Coco S, Bacci A, Pravettoni E, Schenk U, Verderio C, Varoqui H, Erickson JD, Matteoli M. Localization and functional relevance of system A neutral amino acid transporters in cultured hippocampal neurons. J Biol Chem. 2002;277:10467–10473. - PubMed
-
- Ates O, Cayli SR, Gurses I, Karabulut AB, Yucel N, Kocak A, Cakir CO, Yologlu S. Do sodium channel blockers have neuroprotective effect after onset of ischemic insult? Neurol. Res. 2013;29:317–323. - PubMed
-
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–2310. - PubMed
-
- Bae H-J, Lee Y-S, Kang D-W, Gu J-S, Yoon B-W, Roh J-K. Neuroprotective effect of low dose riluzole in gerbil model of transient global ischemia. Neurosci Lett. 2000;294:29–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

