Intratumoral Cancer Cell Intravasation Can Occur Independent of Invasion into the Adjacent Stroma
- PMID: 28423322
- PMCID: PMC5659755
- DOI: 10.1016/j.celrep.2017.03.064
Intratumoral Cancer Cell Intravasation Can Occur Independent of Invasion into the Adjacent Stroma
Abstract
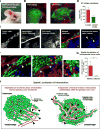
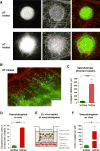
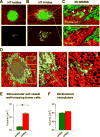
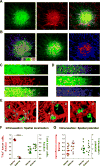
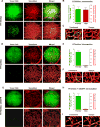
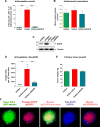
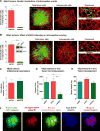
Intravasation, active entry of cancer cells into the circulation, is often considered to be a relatively late event in tumor development occurring after stromal invasion. Here, we provide evidence that intravasation can be initiated early during tumor development and proceed in parallel to or independent of tumor invasion into surrounding stroma. By applying direct and unbiased intravasation-scoring methods to two histologically distinct human cancer types in live-animal models, we demonstrate that intravasation takes place almost exclusively within the tumor core, involves intratumoral vasculature, and does not involve vasculotropic cancer cells invading tumor-adjacent stroma and migrating along tumor-converging blood vessels. Highlighting an additional role for EGFR in cancer, we find that EGFR is required for the development of an intravasation-sustaining intratumoral vasculature. Intratumoral localization of intravasation supports the notion that overt metastases in cancer patients could be initiated much earlier during cancer progression than appreciated within conventional clinical tumor staging systems.
Keywords: EGFR; animal models of cancer; cancer metastasis; cell intravasation; chorioallantoic membrane model; mouse ear tumor model; stromal invasion; tumor angiogenesis; tumor cell migration; tumor invasion.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
EGFR regulates the development and microarchitecture of intratumoral angiogenic vasculature capable of sustaining cancer cell intravasation.Neoplasia. 2015 Aug;17(8):634-49. doi: 10.1016/j.neo.2015.08.002. Neoplasia. 2015. PMID: 26408256 Free PMC article.
-
Chorioallantoic Membrane Microtumor Model to Study the Mechanisms of Tumor Angiogenesis, Vascular Permeability, and Tumor Cell Intravasation.Methods Mol Biol. 2016;1430:283-98. doi: 10.1007/978-1-4939-3628-1_19. Methods Mol Biol. 2016. PMID: 27172961
-
LTBP3 promotes early metastatic events during cancer cell dissemination.Oncogene. 2018 Apr;37(14):1815-1829. doi: 10.1038/s41388-017-0075-1. Epub 2018 Jan 19. Oncogene. 2018. PMID: 29348457 Free PMC article.
-
[Role of the stroma in the initiation and progression of tumors].Orv Hetil. 2015 Nov 8;156(45):1816-23. doi: 10.1556/650.2015.30294. Orv Hetil. 2015. PMID: 26522855 Review. Hungarian.
-
Intravasation as a Key Step in Cancer Metastasis.Biochemistry (Mosc). 2019 Jul;84(7):762-772. doi: 10.1134/S0006297919070071. Biochemistry (Mosc). 2019. PMID: 31509727 Review.
Cited by
-
Lung cancer intravasation-on-a-chip: Visualization and machine learning-assisted automatic quantification.Bioact Mater. 2025 Jun 27;51:858-875. doi: 10.1016/j.bioactmat.2025.06.028. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40678259 Free PMC article.
-
Intravital imaging to study cancer progression and metastasis.Nat Rev Cancer. 2023 Jan;23(1):25-42. doi: 10.1038/s41568-022-00527-5. Epub 2022 Nov 16. Nat Rev Cancer. 2023. PMID: 36385560 Free PMC article. Review.
-
Circulating tumor cell enumeration for improved screening and disease detection of patients with colorectal cancer.Biomed J. 2021 Dec;44(6 Suppl 2):S190-S200. doi: 10.1016/j.bj.2020.09.006. Epub 2020 Sep 30. Biomed J. 2021. PMID: 35292267 Free PMC article.
-
Mutation or not, what directly establishes a neoplastic state, namely cellular immortality and autonomy, still remains unknown and should be prioritized in our research.J Cancer. 2022 Jul 4;13(9):2810-2843. doi: 10.7150/jca.72628. eCollection 2022. J Cancer. 2022. PMID: 35912015 Free PMC article. Review.
-
Adapt and conquer: Metabolic flexibility in cancer growth, invasion and evasion.Mol Metab. 2020 Mar;33:83-101. doi: 10.1016/j.molmet.2019.08.021. Epub 2019 Oct 10. Mol Metab. 2020. PMID: 31668988 Free PMC article. Review.
References
-
- Bobek V, Kolostova K, Pinterova D, Kacprzak G, Adamiak J, Kolodziej J, Boubelik M, Kubecova M, Hoffman RM. A clinically relevant, syngeneic model of spontaneous, highly metastatic B16 mouse melanoma. Anticancer Res. 2010;30:4799–4803. - PubMed
-
- Cochet A, Dygai-Cochet I, Riedinger JM, Humbert O, Berriolo-Riedinger A, Toubeau M, Guiu S, Coutant C, Coudert B, Fumoleau P, et al. (1)(8)F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur J Nucl Med Mol Imaging. 2014;41:428–437. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

