Liberated PKA Catalytic Subunits Associate with the Membrane via Myristoylation to Preferentially Phosphorylate Membrane Substrates
- PMID: 28423323
- PMCID: PMC5481286
- DOI: 10.1016/j.celrep.2017.03.070
Liberated PKA Catalytic Subunits Associate with the Membrane via Myristoylation to Preferentially Phosphorylate Membrane Substrates
Abstract
Protein kinase A (PKA) has diverse functions in neurons. At rest, the subcellular localization of PKA is controlled by A-kinase anchoring proteins (AKAPs). However, the dynamics of PKA upon activation remain poorly understood. Here, we report that elevation of cyclic AMP (cAMP) in neuronal dendrites causes a significant percentage of the PKA catalytic subunit (PKA-C) molecules to be released from the regulatory subunit (PKA-R). Liberated PKA-C becomes associated with the membrane via N-terminal myristoylation. This membrane association does not require the interaction between PKA-R and AKAPs. It slows the mobility of PKA-C and enriches kinase activity on the membrane. Membrane-residing PKA substrates are preferentially phosphorylated compared to cytosolic substrates. Finally, the myristoylation of PKA-C is critical for normal synaptic function and plasticity. We propose that activation-dependent association of PKA-C renders the membrane a unique PKA-signaling compartment. Constrained mobility of PKA-C may synergize with AKAP anchoring to determine specific PKA function in neurons.
Keywords: AMPA/NMDA current radio; PKA; activation-dependent membrane association; cAMP-dependent kinase; diffusion; mEPSC; mobility; myristoylation; synaptic plasticity.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
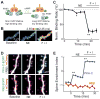
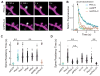
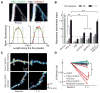



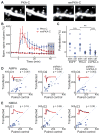
References
-
- Beavo JA, Bechtel PJ, Krebs EG. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974b;38:299–308. - PubMed
-
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

