Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function
- PMID: 28423497
- PMCID: PMC5400560
- DOI: 10.18632/oncotarget.15470
Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function
Abstract
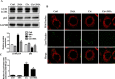
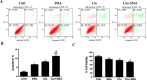
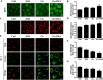
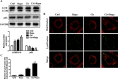
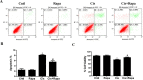
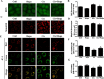
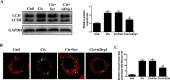
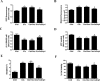
Cisplatin chemotherapy often causes acute kidney injury (AKI) in cancer patients. There is increasing evidence that mitochondrial dysfunction plays an important role in cisplatin-induced nephrotoxicity. Degradation of damaged mitochondria is carried out by mitophagy. Although mitophagy is considered of particular importance in protecting against AKI, little is known of the precise role of mitophagy and its molecular mechanisms during cisplatin-induced nephrotoxicity. Also, evidence that activation of mitophagy improved mitochondrial function is lacking. Furthermore, several evidences have shown that mitochondrial fission coordinates with mitophagy. The aim of this study was to investigate whether activation of mitophagy protects against mitochondrial dysfunction and renal proximal tubular cells injury during cisplatin treatment. The effect of mitochondrial fission on mitophagy was also investigated. In cultured human renal proximal tubular cells, we observed that 3-methyladenine, a pharmacological inhibitor of autophagy, blocked mitophagy and exacerbated cisplatin-induced mitochondrial dysfunction and cells injury. In contrast, autophagy activator rapamycin enhanced mitophagy and protected against the harmful effects of cisplatin on mitochondrial function and cells viability. Suppression of mitochondrial fission by knockdown of its main regulator dynamin-related protein-1 (Drp1) decreased cisplatin-induced mitophagy. Meanwhile, Drp1 suppression protected against cisplatin-induced cells injury by inhibiting mitochondrial dysfunction. Our results provide evidence that Drp1-depedent mitophagy has potential as renoprotective targets for the treatment of cisplatin-induced AKI.
Keywords: AKI; Drp1; cisplatin; mitochondrial dysfunction; mitochondrial fission; mitophagy.
Conflict of interest statement
None.
Figures


Similar articles
-
Inhibition of mitochondrial over-division by (+)-14,15-Dehydrovincamine attenuates cisplatin-induced acute kidney injury via the JNK/Mff pathway.Free Radic Biol Med. 2024 Nov 1;224:190-203. doi: 10.1016/j.freeradbiomed.2024.08.034. Epub 2024 Aug 27. Free Radic Biol Med. 2024. PMID: 39197599
-
PGAM5 regulates PINK1/Parkin-mediated mitophagy via DRP1 in CCCP-induced mitochondrial dysfunction.Toxicol Lett. 2018 Mar 1;284:120-128. doi: 10.1016/j.toxlet.2017.12.004. Epub 2017 Dec 11. Toxicol Lett. 2018. PMID: 29241732
-
Mitophagy Plays a Protective Role in Iodinated Contrast-Induced Acute Renal Tubular Epithelial Cells Injury.Cell Physiol Biochem. 2018;46(3):975-985. doi: 10.1159/000488827. Epub 2018 Apr 19. Cell Physiol Biochem. 2018. PMID: 29680838
-
Dynamin-related protein 1 (Drp1) mediating mitophagy contributes to the pathophysiology of nervous system diseases and brain injury.Histol Histopathol. 2017 Jun;32(6):551-559. doi: 10.14670/HH-11-841. Epub 2016 Nov 10. Histol Histopathol. 2017. PMID: 27830583 Review.
-
[Mechanism of mitochondrial fission - structure and function of Drp1 protein].Postepy Biochem. 2016;62(2):127-137. Postepy Biochem. 2016. PMID: 28132464 Review. Polish.
Cited by
-
Autophagy in kidney homeostasis and disease.Nat Rev Nephrol. 2020 Sep;16(9):489-508. doi: 10.1038/s41581-020-0309-2. Epub 2020 Jul 23. Nat Rev Nephrol. 2020. PMID: 32704047 Free PMC article. Review.
-
Receptor-mediated mitophagy regulates EPO production and protects against renal anemia.Elife. 2021 May 4;10:e64480. doi: 10.7554/eLife.64480. Elife. 2021. PMID: 33942716 Free PMC article.
-
PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury.Cell Death Dis. 2018 Nov 1;9(11):1113. doi: 10.1038/s41419-018-1152-2. Cell Death Dis. 2018. PMID: 30385753 Free PMC article.
-
Andrographolide alleviates Parkinsonism in MPTP-PD mice via targeting mitochondrial fission mediated by dynamin-related protein 1.Br J Pharmacol. 2019 Dec;176(23):4574-4591. doi: 10.1111/bph.14823. Epub 2019 Nov 14. Br J Pharmacol. 2019. PMID: 31389613 Free PMC article.
-
The Emerging Role of Mitophagy in Kidney Diseases.J Life Sci (Westlake Village). 2019 Dec;1(3):13-22. doi: 10.36069/jols/20191203. J Life Sci (Westlake Village). 2019. PMID: 32099974 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

