miR-503-5p confers drug resistance by targeting PUMA in colorectal carcinoma
- PMID: 28423513
- PMCID: PMC5400618
- DOI: 10.18632/oncotarget.15559
miR-503-5p confers drug resistance by targeting PUMA in colorectal carcinoma
Abstract
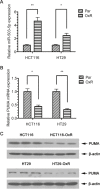
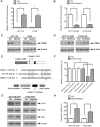
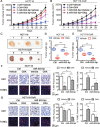
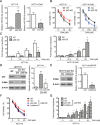
The development of multidrug-resistance (MDR) is a major contributor to death in colorectal carcinoma (CRC). Here, we investigated the possible role of microRNA (miR)-503-5p in drug resistant CRC cells. Unbiased microRNA array screening revealed that miR-503-5p is up-regulated in two oxaliplatin (OXA)-resistant CRC cell lines. Overexpression of miR-503-5p conferred resistance to OXA-induced apoptosis and inhibition of tumor growth in vitro and in vivo through down-regulation of PUMA expression. miR-503-5p knockdown sensitized chemoresistant CRC cells to OXA. Our studies indicated that p53 suppresses miR-503-5p expression and that deletion of p53 upregulates miR-503-5p expression. Inhibition of miR-503-5p in p53 null cells increased their sensitivity to OXA treatment. Importantly, analysis of patient samples showed that expression of miR-503-5p negatively correlates with PUMA in CRC. These results indicate that a p53/miR-503-5p/PUMA signaling axis regulates the CRC response to chemotherapy, and suggest that miR-503-5p plays an important role in the development of MDR in CRC by modulating PUMA expression.
Keywords: PUMA; colorectal carcinoma; miR-503-5p; multidrug-resistance; p53.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Obuch JC, Ahnen DJ. Colorectal Cancer: Genetics is Changing Everything. Gastroenterology clinics of North America. 2016;45:459–476. - PubMed
-
- Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. The New England journal of medicine. 2005;352:476–487. - PubMed
-
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature reviews Drug discovery. 2005;4:307–320. - PubMed
-
- Hector S, Bolanowska-Higdon W, Zdanowicz J, Hitt S, In Pendyala L. vitro studies on the mechanisms of oxaliplatin resistance. Cancer chemotherapy and pharmacology. 2001;48:398–406. - PubMed
-
- Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxidants & redox signaling. 2009;11:2701–2716. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

