Vitamin D and VDR in cancer cachexia and muscle regeneration
- PMID: 28423519
- PMCID: PMC5400623
- DOI: 10.18632/oncotarget.15583
Vitamin D and VDR in cancer cachexia and muscle regeneration
Abstract
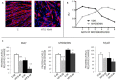
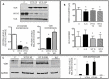
Low circulating levels of vitamin D were associated with decreased muscle strength and physical performance. Along this line, the present study was aimed to investigate: i) the therapeutic potential of vitamin D in cancer-induced muscle wasting; ii) the mechanisms by which vitamin D affects muscle phenotype in tumor-bearing animals.Rats bearing the AH130 hepatoma showed decreased circulating vitamin D compared to control rats, while muscle vitamin D receptor (VDR) mRNA was up-regulated. Both circulating vitamin D and muscle VDR expression increased after vitamin D administration, without exerting appreciable effects on body weight and muscle mass.The effects of vitamin D on muscle cells were studied in C2C12 myocytes. Vitamin D-treated myoblasts did not differentiate properly, fusing only partially and forming multinucleated structures with aberrant shape and low myosin heavy chain content. Vitamin D treatment resulted in VDR overexpression and myogenin down-regulation. Silencing VDR expression in C2C12 cultures abrogated the inhibition of differentiation exerted by vitamin D treatment.These data suggest that VDR overexpression in tumor-bearing animals contributes to muscle wasting by impairing muscle regenerative program. In this regard, attention should be paid when considering vitamin D supplementation to patients affected by chronic pathologies where muscle regeneration may be involved.
Keywords: circulating vitamin D; muscle wasting; myogenin; regeneration; vitamin D receptor.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





References
-
- Muscaritoli M, Molfino A, Lucia S, Rossi Fanelli F. Cachexia: a preventable comorbidity of cancer A T.A.R.G.E.T. approach. Crit Rev Oncol Hematol. 2015;94:251–259. - PubMed
-
- Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–762. - PubMed
-
- Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995;268:E996–1006. - PubMed

