PIF* promotes brain re-myelination locally while regulating systemic inflammation- clinically relevant multiple sclerosis M.smegmatis model
- PMID: 28423529
- PMCID: PMC5400627
- DOI: 10.18632/oncotarget.15662
PIF* promotes brain re-myelination locally while regulating systemic inflammation- clinically relevant multiple sclerosis M.smegmatis model
Abstract
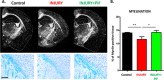
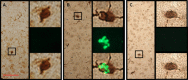
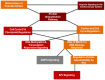
Neurologic disease diagnosis and treatment is challenging. Multiple Sclerosis (MS) is a demyelinating autoimmune disease with few clinical forms and uncertain etiology. Current studies suggest that it is likely caused by infection(s) triggering a systemic immune response resulting in antigen/non-antigen-related autoimmune response in central nervous system (CNS). New therapeutic approaches are needed. Secreted by viable embryos, PreImplantation Factor (PIF) possesses a local and systemic immunity regulatory role. Synthetic PIF (PIF) duplicates endogenous peptide's protective effect in pre-clinical autoimmune and transplantation models. PIF protects against brain hypoxia-ischemia by directly targeting microglia and neurons. In chronic experimental autoimmune encephalitis (EAE) model PIF reverses paralysis while promoting neural repair. Herein we report that PIF directly promotes brain re-myelination and reverses paralysis in relapsing remitting EAE MS model. PIF crosses the blood-brain barrier targeting microglia. Systemically, PIF decreases pro-inflammatory IL23/IL17 cytokines, while preserving CNS-specific T-cell repertoire. Global brain gene analysis revealed that PIF regulates critical Na+/K+/Ca++ ions, amino acid and glucose transport genes expression. Further, PIF modulates oxidative stress, DNA methylation, cell cycle regulation, and protein ubiquitination while regulating multiple genes. In cultured astrocytes, PIF promotes BDNF-myelin synthesis promoter and SLC2A1 (glucose transport) while reducing deleterious E2F5, and HSP90ab1 (oxidative stress) genes expression. In cultured microglia, PIF increases anti-inflammatory IL10 while reducing pro-inflammatory IFNγ expression. Collectively, PIF promotes brain re-myelination and neuroprotection in relapsing remitting EAE MS model. Coupled with ongoing, Fast-Track FDA approved clinical trial, NCT#02239562 (immune disorder), current data supports PIF's translation for neurodegenerative disorders therapy.
Keywords: M. smegmatis bacteria; RR-EAE clinically-relevant model; neuroprotection; neuroregeneration; preImplantation factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Immune regulatory and neuroprotective properties of preimplantation factor: From newborn to adult.Pharmacol Ther. 2015 Dec;156:10-25. doi: 10.1016/j.pharmthera.2015.10.008. Epub 2015 Nov 3. Pharmacol Ther. 2015. PMID: 26546485 Review.
-
PIF direct immune regulation: Blocks mitogen-activated PBMCs proliferation, promotes TH2/TH1 bias, independent of Ca(2+).Immunobiology. 2015 Jul;220(7):865-75. doi: 10.1016/j.imbio.2015.01.010. Epub 2015 Jan 31. Immunobiology. 2015. PMID: 25766203
-
Distinct pathological patterns in relapsing-remitting and chronic models of experimental autoimmune enchephalomyelitis and the neuroprotective effect of glatiramer acetate.J Autoimmun. 2011 Nov;37(3):228-41. doi: 10.1016/j.jaut.2011.06.003. Epub 2011 Jul 14. J Autoimmun. 2011. PMID: 21752599
-
Preimplantation factor (PIF*) reverses neuroinflammation while promoting neural repair in EAE model.J Neurol Sci. 2012 Jan 15;312(1-2):146-57. doi: 10.1016/j.jns.2011.07.050. Epub 2011 Oct 13. J Neurol Sci. 2012. PMID: 21996270
-
FGF/FGFR Pathways in Multiple Sclerosis and in Its Disease Models.Cells. 2021 Apr 13;10(4):884. doi: 10.3390/cells10040884. Cells. 2021. PMID: 33924474 Free PMC article. Review.
Cited by
-
Preimplantation factor modulates oligodendrocytes by H19-induced demethylation of NCOR2.JCI Insight. 2021 Oct 22;6(20):e132335. doi: 10.1172/jci.insight.132335. JCI Insight. 2021. PMID: 34676826 Free PMC article.
-
Synthetic PreImplantation Factor (sPIF) reduces inflammation and prevents preterm birth.PLoS One. 2020 Jun 8;15(6):e0232493. doi: 10.1371/journal.pone.0232493. eCollection 2020. PLoS One. 2020. PMID: 32511256 Free PMC article.
-
Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose Trial of Synthetic Preimplantation Factor in Autoimmune Hepatitis.Hepatol Commun. 2018 Sep 26;2(10):1235-1246. doi: 10.1002/hep4.1239. eCollection 2018 Oct. Hepatol Commun. 2018. PMID: 30411073 Free PMC article.
-
Pregnancy-Induced Changes in microRNA Expression in Multiple Sclerosis.Front Immunol. 2021 Jan 28;11:552101. doi: 10.3389/fimmu.2020.552101. eCollection 2020. Front Immunol. 2021. PMID: 33584638 Free PMC article.
-
Synthetic PreImplantation Factor (sPIF) induces posttranslational protein modification and reverses paralysis in EAE mice.Sci Rep. 2019 Oct 2;9(1):12876. doi: 10.1038/s41598-019-48473-x. Sci Rep. 2019. PMID: 31578341 Free PMC article.
References
-
- Barnea ER, Almogi-Hazan O, Or R, Mueller M, Ria F, Weiss L, Paidas MJ. Immune regulatory and neuroprotective properties of preimplantation factor: From newborn to adult. Pharmacol Ther. 2015;156:10–25. - PubMed
-
- de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59:1241–1248. - PubMed
-
- Barnea ER, Rambaldi M, Paidas MJ, Mecacci F. Reproduction and autoimmune disease: important translational implications from embryo-maternal interaction. Immunotherapy. 2013;5:769–780. - PubMed
-
- Paidas MJ, Annunziato J, Romano M, Weiss L, Or R, Barnea ER. Pregnancy and multiple sclerosis (MS): a beneficial association. Possible therapeutic application of embryo-specific pre-implantation factor (PIF*) Am J Reprod Immunol. 2012;68:456–464. - PubMed
-
- Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol. 2002;169:1084–1091. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

