Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study
- PMID: 28423535
- PMCID: PMC5400633
- DOI: 10.18632/oncotarget.15746
Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study
Abstract
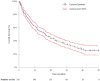
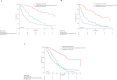
Overall survival (OS) with the anaplastic lymphoma kinase (ALK) inhibitor (ALKi) crizotinib in a large population of unselected patients with ALK-positive non-small-cell lung cancer (NSCLC) is not documented. We sought to assess OS with crizotinib in unselected ALK-positive NSCLC patients and whether post-progression systemic treatments affect survival outcomes.ALK-positive NSCLC patients receiving crizotinib in French expanded access programs or as approved drug were enrolled. We collected clinical and survival data, RECIST-defined progressive disease (PD) and post-PD systemic treatment efficacy. We performed multivariable analysis of OS from crizotinib initiation and PD under crizotinib.At time of analysis, 209 (65.7%) of the 318 included patients had died. Median OS with crizotinib was 16.6 months. The line of crizotinib therapy did not impact survival outcomes. Of the 263 patients with PD, 105 received best supportive care, 74 subsequent drugs other than next-generation ALKi and 84 next-generation ALKi. Next-generation ALKi treatment correlated with better survival outcomes in multivariate analysis. These patients had a median post-PD survival of 25.0 months and median OS from metastatic disease diagnosis of 89.6 months.Unselected ALK-positive NSCLC patients achieve good survival outcomes with crizotinib therapy. Next-generation ALKi may provide survival improvement after PD under crizotinib.
Keywords: ALK; alectinib; ceritinib; crizotinib; lung cancer.
Conflict of interest statement
MD has received research funding from Novartis. He has been reimbursed for travel, accommodation, and/or other expenses by Novartis, Pfizer and Roche.
BB has received research funding from Novartis.
JC has received research funding from Novartis and Pfizer. He has served as a consultant (advisory board) for Novartis, Pfizer and Roche.
MP served as a consultant (advisory board) for Novartis, Pfizer, Roche and Lilly.
BM has served as a consultant (advisory board) for Novartis, Pfizer, Bristol-Myers Squibb, MSD and Lilly.
ED has received research funding from Roche. He has served as a consultant (advisory board) for Novartis, Pfizer, Roche and Lilly.
CAV has served as a consultant (advisory board) for Pfizer.
LM has been reimbursed for travel, accommodation, and/or other expenses by Pfizer.
RV has served as a consultant (advisory board) for Boehringer Ingelheim, Astra-Zeneca and Bristol-Myers Squibb.
ABC has served as a consultant (advisory board) for Novartis, Pfizer and Roche.
DMS has received research funding from Pfizer. He has served as a consultant (advisory board) for Novartis, Pfizer and Roche.
All remaining authors (LBG, RD, JH, JO, AMF, FG, PBR, AL, PM, FM) have declared no conflicts of interest.
Figures

References
-
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. - PubMed
-
- Barlesi F, Mazieres J, Merlio J-P, Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I, Westeel V, Escande F, Monnet I, Lemoine A, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–26. - PubMed
-
- Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. - PubMed
-
- Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn M-J, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu Y-L, Thomas M, O’Byrne KJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. - PubMed
-
- Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou S-HI, Butaney M, Salgia R, Maki RG, Varella-Garcia M, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

