The cyclin-like protein, SPY1, regulates the ERα and ERK1/2 pathways promoting tamoxifen resistance
- PMID: 28423577
- PMCID: PMC5410308
- DOI: 10.18632/oncotarget.15578
The cyclin-like protein, SPY1, regulates the ERα and ERK1/2 pathways promoting tamoxifen resistance
Abstract
The Ras/Raf/MEK/ERK pathway conveys growth factor and mitogen signalling to control the phosphorylation of a plethora of substrates regulating proliferation, survival, and migration. The Ras signalling pathway is frequently associated with poor prognosis and drug resistance in various cancers including those of the blood, breast and prostate. Activation of the downstream effector ERK does not always occur via a linear cascade of events; complicating the targeting of this pathway therapeutically. This work describes a novel positive feedback loop where the cell cycle regulatory factor Spy1 (RINGO; gene SPDYA) activates ERK1/2 in a MEK-independent fashion. Spy1 was originally isolated for the ability to stimulate Xenopus oocyte maturation via a MAPK-signalling pathway and is known to override apoptosis triggered by the DNA damage response. We demonstrate that mammalian Spy1-mediated ERK activation increases ligand-independent phosphorylation and activation of estrogen receptor α, correlating with a decrease in tamoxifen sensitivity. This could define a novel druggable mechanism driving proliferation and resistance in select cancers.
Keywords: Cdk; cell cycle; cyclin; estrogen; tamoxifen.
Conflict of interest statement
We have no conflicts to report.
Figures

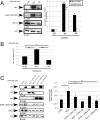
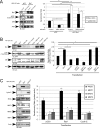

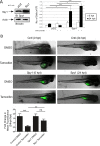
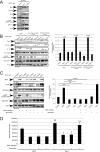
Similar articles
-
Estrogen receptor α is the major driving factor for growth in tamoxifen-resistant breast cancer and supported by HER/ERK signaling.Breast Cancer Res Treat. 2013 May;139(1):71-80. doi: 10.1007/s10549-013-2485-2. Epub 2013 Apr 23. Breast Cancer Res Treat. 2013. PMID: 23609470
-
Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells.J Ethnopharmacol. 2011 Oct 11;137(3):1283-90. doi: 10.1016/j.jep.2011.07.056. Epub 2011 Aug 4. J Ethnopharmacol. 2011. PMID: 21840388
-
Mechanisms of Gefitinib-mediated reversal of tamoxifen resistance in MCF-7 breast cancer cells by inducing ERα re-expression.Sci Rep. 2015 Feb 3;5:7835. doi: 10.1038/srep07835. Sci Rep. 2015. PMID: 25644501 Free PMC article.
-
MAP kinase/estrogen receptor cross-talk enhances estrogen-mediated signaling and tumor growth but does not confer tamoxifen resistance.Oncogene. 2002 Jun 6;21(25):4000-8. doi: 10.1038/sj.onc.1205506. Oncogene. 2002. PMID: 12037682
-
Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance.J Biol Chem. 2001 Mar 30;276(13):9817-24. doi: 10.1074/jbc.M010840200. Epub 2001 Jan 3. J Biol Chem. 2001. PMID: 11139588
Cited by
-
Atypical cell cycle regulation promotes mammary stem cell expansion during mammary development and tumourigenesis.Breast Cancer Res. 2024 Jun 28;26(1):106. doi: 10.1186/s13058-024-01862-1. Breast Cancer Res. 2024. PMID: 38943151 Free PMC article.
-
The atypical CDK activator RingoA/Spy1 regulates exit from quiescence in neural stem cells.iScience. 2023 Feb 14;26(3):106202. doi: 10.1016/j.isci.2023.106202. eCollection 2023 Mar 17. iScience. 2023. PMID: 36876138 Free PMC article.
-
Progress in the Understanding of the Mechanism of Tamoxifen Resistance in Breast Cancer.Front Pharmacol. 2020 Dec 9;11:592912. doi: 10.3389/fphar.2020.592912. eCollection 2020. Front Pharmacol. 2020. PMID: 33362547 Free PMC article. Review.
-
Targeting Pin1 by All-Trans Retinoic Acid (ATRA) Overcomes Tamoxifen Resistance in Breast Cancer via Multifactorial Mechanisms.Front Cell Dev Biol. 2019 Dec 6;7:322. doi: 10.3389/fcell.2019.00322. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31867329 Free PMC article.
-
The Role of Speedy/RINGO Protein in Breast Cancer as a Future Biomarker.Cancer Diagn Progn. 2024 May 3;4(3):209-213. doi: 10.21873/cdp.10310. eCollection 2024 May-Jun. Cancer Diagn Progn. 2024. PMID: 38707716 Free PMC article. Review.
References
-
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. - PubMed
-
- Hackshaw A, Roughton M, Forsyth S, Monson K, Reczko K, Sainsbury R, Baum M. Long-term benefits of 5 years of tamoxifen: 10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J Clin Oncol. 2011;29:1657–1663. - PubMed
-
- Barnes EA, Porter LA, Lenormand JL, Dellinger RW, Donoghue DJ. Human Spy1 promotes survival of mammalian cells following DNA damage. Cancer Res. 2003;63:3701–3707. - PubMed
-
- Gastwirt RF, Slavin DA, McAndrew CW, Donoghue DJ. Spy1 expression prevents normal cellular responses to DNA damage: inhibition of apoptosis and checkpoint activation. J Biol Chem. 2006;281:35425–35435. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

