The stem cell/cancer stem cell marker ALDH1A3 regulates the expression of the survival factor tissue transglutaminase, in mesenchymal glioma stem cells
- PMID: 28423611
- PMCID: PMC5410226
- DOI: 10.18632/oncotarget.16479
The stem cell/cancer stem cell marker ALDH1A3 regulates the expression of the survival factor tissue transglutaminase, in mesenchymal glioma stem cells
Abstract
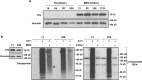
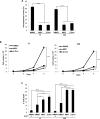
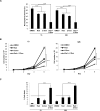
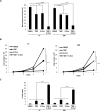
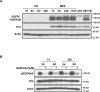
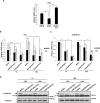
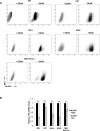
Tissue transglutaminase (tTG), a dual-function enzyme with GTP-binding and acyltransferase activities, has been implicated in the survival and chemotherapy resistance of aggressive cancer cells and cancer stem cells, including glioma stem cells (GSCs). Using a model system comprising two distinct subtypes of GSCs referred to as proneural (PN) and mesenchymal (MES), we find that the phenotypically aggressive and radiation therapy-resistant MES GSCs exclusively express tTG relative to PN GSCs. As such, the self-renewal, proliferation, and survival of these cells was sensitive to treatment with tTG inhibitors, with a benefit being observed when combined with the standard of care for high grade gliomas (i.e. radiation or temozolomide). Efforts to understand the molecular drivers of tTG expression in MES GSCs revealed an unexpected link between tTG and a common marker for stem cells and cancer stem cells, Aldehyde dehydrogenase 1A3 (ALDH1A3). ALDH1A3, as well as other members of the ALDH1 subfamily, can function in cells as a retinaldehyde dehydrogenase to generate retinoic acid (RA) from retinal. We show that the enzymatic activity of ALDH1A3 and its product, RA, are necessary for the observed expression of tTG in MES GSCs. Additionally, the ectopic expression of ALDH1A3 in PN GSCs is sufficient to induce the expression of tTG in these cells, further demonstrating a causal link between ALDH1A3 and tTG. Together, these findings ascribe a novel function for ALDH1A3 in an aggressive GSC phenotype via the up-regulation of tTG, and suggest the potential for a similar role by ALDH1 family members across cancer types.
Keywords: aldehyde dehydrogenase; cancer stem cells; glioblastoma; retinoic acid; tissue transglutaminase.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3.Proc Natl Acad Sci U S A. 2013 May 21;110(21):8644-9. doi: 10.1073/pnas.1221478110. Epub 2013 May 6. Proc Natl Acad Sci U S A. 2013. PMID: 23650391 Free PMC article. Clinical Trial.
-
ALDH1A3: A Marker of Mesenchymal Phenotype in Gliomas Associated with Cell Invasion.PLoS One. 2015 Nov 17;10(11):e0142856. doi: 10.1371/journal.pone.0142856. eCollection 2015. PLoS One. 2015. PMID: 26575197 Free PMC article.
-
ALDH1A3 in CSCs.Aging (Albany NY). 2017 May 3;9(5):1351-1352. doi: 10.18632/aging.101236. Aging (Albany NY). 2017. PMID: 28476022 Free PMC article. No abstract available.
-
Stem cell signature in glioblastoma: therapeutic development for a moving target.J Neurosurg. 2015 Feb;122(2):324-30. doi: 10.3171/2014.9.JNS132253. Epub 2014 Nov 14. J Neurosurg. 2015. PMID: 25397368 Review.
-
ALDH1A3, a metabolic target for cancer diagnosis and therapy.Int J Cancer. 2016 Sep 1;139(5):965-75. doi: 10.1002/ijc.30091. Epub 2016 Apr 4. Int J Cancer. 2016. PMID: 26991532 Review.
Cited by
-
Cellular Components of the Tumor Environment in Gliomas-What Do We Know Today?Biomedicines. 2023 Dec 20;12(1):14. doi: 10.3390/biomedicines12010014. Biomedicines. 2023. PMID: 38275375 Free PMC article. Review.
-
ALDH1A3 Segregated Expression and Nucleus-Associated Proteasomal Degradation Are Common Traits of Glioblastoma Stem Cells.Biomedicines. 2021 Dec 22;10(1):7. doi: 10.3390/biomedicines10010007. Biomedicines. 2021. PMID: 35052687 Free PMC article.
-
Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells.Stem Cells Int. 2019 Jan 13;2019:3904645. doi: 10.1155/2019/3904645. eCollection 2019. Stem Cells Int. 2019. PMID: 30733805 Free PMC article. Review.
-
Aldehyde Dehydrogenase 2 as a Therapeutic Target in Oxidative Stress-Related Diseases: Post-Translational Modifications Deserve More Attention.Int J Mol Sci. 2022 Feb 28;23(5):2682. doi: 10.3390/ijms23052682. Int J Mol Sci. 2022. PMID: 35269824 Free PMC article. Review.
-
TRPM7 Induces Tumorigenesis and Stemness Through Notch Activation in Glioma.Front Pharmacol. 2020 Dec 14;11:590723. doi: 10.3389/fphar.2020.590723. eCollection 2020. Front Pharmacol. 2020. PMID: 33381038 Free PMC article.
References
-
- Antonyak MA, Singh US, Lee DA, Boehm JE, Combs C, Zgola MM, Page RL, Cerione RA. Effects of tissue transglutaminase on retinoic acid-induced cellular differentiation and protection against ap optosis. The Journal of Biological Chemistry. 2001;276:33582–33587. - PubMed
-
- Antonyak MA, Miller AM, Jansen JM, Boehm JE, Balkman CE, Wakshlag JJ, Page RL, Cerione RA. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. The Journal of Biological Chemistry. 2004;279:41461–41467. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

