Inhibition activity of a disulfide-stabilized diabody against basic fibroblast growth factor in lung cancer
- PMID: 28423625
- PMCID: PMC5386754
- DOI: 10.18632/oncotarget.15556
Inhibition activity of a disulfide-stabilized diabody against basic fibroblast growth factor in lung cancer
Abstract
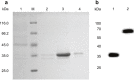
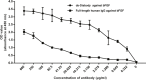
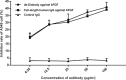
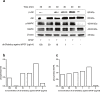
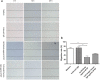
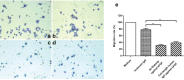
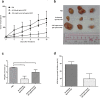
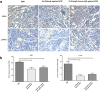
The over-expression of basic fibroblast growth factor (bFGF) plays a crucial role in the development, invasion and metastasis of lung cancer. Therefore, neutralizing antibodies against bFGF may inhibit the growth of lung cancer. In this study, a Disulfide-stabilized diabody (ds-Diabody) against bFGF was constructed by site-directed mutation and overlap extension PCR (SOE-PCR) at the position of VH44 and VL100 in the scFv. The ds-Diabody was constructed and expressed in Pichia pastoris. We found that the ds-Diabody against bFGF could efficiently suppress the proliferation, migration and invasion of human lung cancer A549 cells in vitro. Moreover, in A549 cells, the ds-Diabody against bFGF could inhibit bFGF-induced activation of downstream signaling regulators, such as phospho-Akt and phospho-MAPK. In the nude mouse xenograft model of lung cancer, the ds-Diabody against bFGF could significantly inhibit tumor growth and decrease the densities of micro-vessels and lymphatic vessels in tumor tissue. Our data indicate that the ds-Diabody against bFGF could effectively suppress the lung cancer growth through blockade of bFGF signaling pathway and inhibition of tumor angiogenesis, which may make it a potential therapeutic candidate antibody drug for human lung cancer therapy.
Keywords: angiogenesis; bFGF; ds-diabody; lung cancer; lymphangiogenesis.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

Similar articles
-
Construction of a disulfide-stabilized diabody against fibroblast growth factor-2 and the inhibition activity in targeting breast cancer.Cancer Sci. 2016 Aug;107(8):1141-50. doi: 10.1111/cas.12981. Epub 2016 Jul 26. Cancer Sci. 2016. PMID: 27251178 Free PMC article.
-
Fine epitope mapping of a human disulphide-stabilized diabody against fibroblast growth factor-2.J Biochem. 2019 Jun 1;165(6):487-495. doi: 10.1093/jb/mvy122. J Biochem. 2019. PMID: 30597085
-
Humanized disulfide-stabilized diabody against fibroblast growth factor-2 inhibits PD-L1 expression and epithelial-mesenchymal transition in hepatoma cells through STAT3.IUBMB Life. 2023 Nov;75(11):957-968. doi: 10.1002/iub.2766. Epub 2023 Jul 25. IUBMB Life. 2023. PMID: 37489553
-
Development of angiogenesis inhibitors for clinical applications.Trends Pharmacol Sci. 1990 Nov;11(11):457-61. doi: 10.1016/0165-6147(90)90127-t. Trends Pharmacol Sci. 1990. PMID: 1702563 Review.
-
Far beyond anti-angiogenesis: Benefits for anti-basicFGF therapy in cancer.Biochim Biophys Acta Mol Cell Res. 2022 Jul;1869(7):119253. doi: 10.1016/j.bbamcr.2022.119253. Epub 2022 Mar 5. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35259425 Review.
Cited by
-
Lymphatic transport in anti-tumor immunity and metastasis.J Exp Med. 2025 Mar 3;222(3):e20231954. doi: 10.1084/jem.20231954. Epub 2025 Feb 19. J Exp Med. 2025. PMID: 39969537 Review.
-
Production of Therapeutic Single-Chain Variable Fragments (ScFv) in Pichia pastoris.Methods Mol Biol. 2022;2313:151-167. doi: 10.1007/978-1-0716-1450-1_8. Methods Mol Biol. 2022. PMID: 34478136
-
The lymphatic vasculature: An active and dynamic player in cancer progression.Med Res Rev. 2022 Jan;42(1):576-614. doi: 10.1002/med.21855. Epub 2021 Sep 5. Med Res Rev. 2022. PMID: 34486138 Free PMC article. Review.
-
Specific Antibody Fragment Ligand Traps Blocking FGF1 Activity.Int J Mol Sci. 2018 Aug 21;19(9):2470. doi: 10.3390/ijms19092470. Int J Mol Sci. 2018. PMID: 30134556 Free PMC article.
References
-
- Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437:199–213. - PubMed
-
- Zhang Q, Lao X, Huang J, Zhu Z, Pang L, Tang Y, Song Q, Huang J, Deng J, Deng N. Soluble production and function of vascular endothelial growth factor/basic fibroblast growth factor complex peptide. Biotechnology progress. 2015;31:194–203. - PubMed
-
- Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine & Growth Factor Reviews. 2005;16:159–78. - PubMed
-
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocrine reviews. 1997;18:26–45. - PubMed
-
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine & Growth Factor Reviews. 2005;16:139–49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

