HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E
- PMID: 28423638
- PMCID: PMC5386727
- DOI: 10.18632/oncotarget.15773
HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E
Abstract
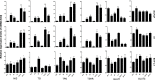
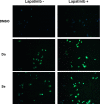
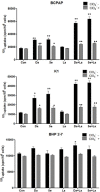
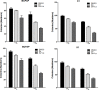
Redifferentiation therapy with BRAF/MEK inhibitors to facilitate treatment with radioiodine represents a good choice for radioiodine-refractory differentiated thyroid carcinoma, but recent initial clinical outcomes were modest. MAPK rebound caused by BRAF/MEK inhibitors-induced activation of HER2/HER3 is a resistance mechanism, and combination with HER inhibitor to prevent MAPK rebound may sensitize BRAFV600E-mutant thyroid cancer cells to redifferentiation therapy. To evaluate if inhibiting both BRAF/MEK and HER can produce stronger redifferetiation effect, we tested the effects of BRAF/MEK inhibitor dabrafenib/selumetinib alone or in combination with HER inhibitor lapatinib on the expression and function of iodine- and glucose-handling genes in BRAFV600E-positive BCPAP and K1 cells, using BHP 2-7 cells harboring RET/PTC1 rearrangement as control. Herein, we showed that lapatinib prevented MAPK rebound and sensitized BRAFV600E-positive papillary thyroid cancer cells to BRAF/MEK inhibitors. Dabrafenib/selumetinib alone increased iodine-uptake and toxicity and suppressed glucose-metablism in BRAFV600E-positive papillary thyroid cancer cells. When lapatinib was added, more significant effects on iodine- and glucose-handling gene expression, cell membrane location of sodium/iodine symporter as well as radioiodine uptake and toxicity were observed. Thus, combined therapy using HER inhibitor and BRAF/MEK inhibitor presented more significant redifferentiation effect on papillary thyroid cancer cells harboring BRAFV600E than BRAF/MEK inhibitor alone. In vivo and clinical studies assessing such combined targeted redifferentiation strategy were warranted.
Keywords: dabrafenib; glucose; iodine; papillary thyroid cancer; redifferentiation.
Conflict of interest statement
None of the authors has any conflict of interest to declare.
Figures


Similar articles
-
Mitogen-Activated Protein Kinase Pathway Inhibition for Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer: An Evolving Protocol.Thyroid. 2019 Nov;29(11):1634-1645. doi: 10.1089/thy.2019.0143. Thyroid. 2019. PMID: 31637953
-
Combined tazemetostat and MAPKi enhances differentiation of papillary thyroid cancer cells harbouring BRAFV600E by synergistically decreasing global trimethylation of H3K27.J Cell Mol Med. 2020 Mar;24(6):3336-3345. doi: 10.1111/jcmm.15007. Epub 2020 Jan 22. J Cell Mol Med. 2020. PMID: 31970877 Free PMC article.
-
The MEK1/2 Inhibitor AZD6244 Sensitizes BRAF-Mutant Thyroid Cancer to Vemurafenib.Med Sci Monit. 2018 May 8;24:3002-3010. doi: 10.12659/MSM.910084. Med Sci Monit. 2018. Retraction in: Med Sci Monit. 2022 Mar 07;28:e936571. doi: 10.12659/MSM.936571. PMID: 29737325 Free PMC article. Retracted.
-
The Significance of BRAFV600E Mutation in Thyroid Cancer in Terms of Novel Targeted Therapies - Overview of Current Knowledge and Studies.Klin Onkol. 2018 Fall;31(5):339-344. doi: 10.14735/amko2018339. Klin Onkol. 2018. PMID: 30541319 Review. English.
-
Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations.Cancer. 2005 Jun 1;103(11):2261-8. doi: 10.1002/cncr.21073. Cancer. 2005. PMID: 15880523 Review.
Cited by
-
MAPK Pathway Inhibitors in Thyroid Cancer: Preclinical and Clinical Data.Cancers (Basel). 2023 Jan 24;15(3):710. doi: 10.3390/cancers15030710. Cancers (Basel). 2023. PMID: 36765665 Free PMC article. Review.
-
Combinatorial Therapies in Thyroid Cancer: An Overview of Preclinical and Clinical Progresses.Cells. 2020 Mar 30;9(4):830. doi: 10.3390/cells9040830. Cells. 2020. PMID: 32235612 Free PMC article. Review.
-
Emerging Therapies for Radioactive Iodine Refractory Thyroid Cancer.Curr Treat Options Oncol. 2020 Feb 11;21(3):18. doi: 10.1007/s11864-020-0714-6. Curr Treat Options Oncol. 2020. PMID: 32048061 Review.
-
Telomerase reverse transcriptase induced thyroid carcinoma cell proliferation through PTEN/AKT signaling pathway.Mol Med Rep. 2018 Aug;18(2):1345-1352. doi: 10.3892/mmr.2018.9119. Epub 2018 Jun 1. Mol Med Rep. 2018. PMID: 29901196 Free PMC article.
-
Identifying and analyzing the key genes shared by papillary thyroid carcinoma and Hashimoto's thyroiditis using bioinformatics methods.Front Endocrinol (Lausanne). 2023 May 31;14:1140094. doi: 10.3389/fendo.2023.1140094. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37324256 Free PMC article.
References
-
- Scott E, Learoyd D, Clifton-Bligh RJ. Therapeutic options in papillary thyroid carcinoma: current guidelines and future perspectives. Future Oncol. 2016;12:2603–2613. - PubMed
-
- Vaisman F, Carvalho DP, Vaisman M. A new appraisal of iodine refractory thyroid cancer. Endocr Relat Cancer. 2015;22:R301–310. - PubMed
-
- Grabellus F, Nagarajah J, Bockisch A, Schmid KW, Sheu SY. Glucose transporter 1 expression, tumor proliferation, and iodine/glucose uptake in thyroid cancer with emphasis on poorly differentiated thyroid carcinoma. Clin Nucl Med. 2012;37:121–127. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

