Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade
- PMID: 28423670
- PMCID: PMC5458249
- DOI: 10.18632/oncotarget.15992
Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade
Abstract
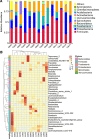
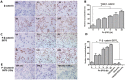
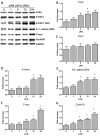
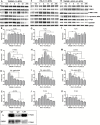
The underlying mechanism of Fusobacterium nucleatum (Fn) in the carcinogenesis of colorectal cancer (CRC) is poorly understood. Here, we examined Fn abundance in CRC tissues, as well as β-catenin, TLR4 and PAK1 protein abundance in Fn positive and Fn negative CRCs. Furthermore, we isolated a strain of Fn (F01) from a CRC tissue and examined whether Fn (F01) infection of colon cancer cells activated β-catenin signaling via the TLR4/P-PAK1/P-β-catenin S675 cascade. Invasive Fn was abundant in 62.2% of CRC tissues. TLR4, PAK1 and nuclear β-catenin proteins were more abundant within Fn-positive over Fn-negative CRCs (P < 0.05). Fn and its lipopolysaccharide induced a significant increase in TLR4/P-PAK1/P-β-catenin S675/C-myc/CyclinD1 protein abundance, as well as in the nuclear translocation of β-catenin. Furthermore, inhibition of TLR4 or PAK1 prior to challenge with Fn significantly decreased protein abundance of P-β-catenin S675, C-myc and Cyclin D1, as well as nuclear β-catenin accumulation. Inhibition of TLR4 significantly decreased P-PAK1 protein abundance, and for the first time, we observed an interaction between TLR4 and P-PAK1 using immunoprecipitation. Our data suggest that invasive Fn activates β-catenin signaling via a TLR4/P-PAK1/P-β-catenin S675 cascade in CRC. Furthermore, TLR4 and PAK1 could be potential pharmaceutical targets for the treatment of Fn-related CRCs.
Keywords: Fusobacterium nucleatum; colorectal cancer; p21-activated kinase 1; toll-like receptor 4; β-catenin signaling.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis in Mice via a Toll-Like Receptor 4/p21-Activated Kinase 1 Cascade.Dig Dis Sci. 2018 May;63(5):1210-1218. doi: 10.1007/s10620-018-4999-2. Epub 2018 Mar 5. Dig Dis Sci. 2018. PMID: 29508166
-
Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-κB/S100A9 Cascade.Front Immunol. 2021 May 21;12:658681. doi: 10.3389/fimmu.2021.658681. eCollection 2021. Front Immunol. 2021. PMID: 34093546 Free PMC article.
-
Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism.Cancer Immunol Immunother. 2018 Oct;67(10):1635-1646. doi: 10.1007/s00262-018-2233-x. Epub 2018 Aug 18. Cancer Immunol Immunother. 2018. PMID: 30121899 Free PMC article.
-
Fusobacterium nucleatum modulates the Wnt/β-catenin pathway in colorectal cancer development.Int J Biol Macromol. 2025 Apr;299:140196. doi: 10.1016/j.ijbiomac.2025.140196. Epub 2025 Jan 21. Int J Biol Macromol. 2025. PMID: 39848378 Review.
-
Fusobacterium nucleatum: an emerging bug in colorectal tumorigenesis.Eur J Cancer Prev. 2015 Sep;24(5):373-85. doi: 10.1097/CEJ.0000000000000116. Eur J Cancer Prev. 2015. PMID: 25569450 Review.
Cited by
-
The Oncobiome in Gastroenteric and Genitourinary Cancers.Int J Mol Sci. 2022 Aug 26;23(17):9664. doi: 10.3390/ijms23179664. Int J Mol Sci. 2022. PMID: 36077063 Free PMC article. Review.
-
Fusobacterium nucleatum: Unraveling its potential role in gastric carcinogenesis.World J Gastroenterol. 2024 Sep 21;30(35):3972-3984. doi: 10.3748/wjg.v30.i35.3972. World J Gastroenterol. 2024. PMID: 39351058 Free PMC article. Review.
-
Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism.Front Cell Infect Microbiol. 2022 Nov 29;12:1020583. doi: 10.3389/fcimb.2022.1020583. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36523635 Free PMC article. Review.
-
Fusobacterium nucleatum: a new player in regulation of cancer development and therapeutic response.Cancer Drug Resist. 2022 May 12;5(2):436-450. doi: 10.20517/cdr.2021.144. eCollection 2022. Cancer Drug Resist. 2022. PMID: 35800370 Free PMC article. Review.
-
Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis.J Biomed Sci. 2018 Nov 9;25(1):79. doi: 10.1186/s12929-018-0483-8. J Biomed Sci. 2018. PMID: 30413188 Free PMC article. Review.
References
-
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–15. - PMC - PubMed
-
- Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–08. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

