Experience with precision genomics and tumor board, indicates frequent target identification, but barriers to delivery
- PMID: 28423702
- PMCID: PMC5432324
- DOI: 10.18632/oncotarget.16057
Experience with precision genomics and tumor board, indicates frequent target identification, but barriers to delivery
Abstract
Background: The ability to analyze the genomics of malignancies has opened up new possibilities for off-label targeted therapy in cancers that are refractory to standard therapy. At Mayo Clinic these efforts are organized through the Center for Individualized Medicine (CIM).
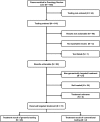
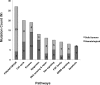
Results: Prior to GTB, datasets were analyzed and integrated by a team of bioinformaticians and cancer biologists. Therapeutically actionable mutations were identified in 65% (92/141) of the patients tested with 32% (29/92) receiving genomically targeted therapy with FDA approved drugs or in an independent clinical trial with 45% (13/29) responding. Standard of care (SOC) options were continued by 15% (14/92) of patients tested before exhausting SOC options, with 71% (10/14) responding to treatment. Over 35% (34/92) of patients with actionable targets were not treated with 65% (22/34) choosing comfort measures or passing away.
Materials and methods: Patients (N = 165) were referred to the CIM Clinic between October 2012 and December 2015. All patients received clinical genomic panel testing with selected subsets receiving array comparative genomic hybridization and clinical whole exome sequencing to complement and validate panel findings. A genomic tumor board (GTB) reviewed results and, when possible, developed treatment recommendations.
Conclusions: Treatment decisions driven by tumor genomic analysis can lead to significant clinical benefit in a minority of patients. The success of genomically driven therapy depends both on access to drugs and robustness of bioinformatics analysis. While novel clinical trial designs are increasing the utility of genomic testing, robust data sharing of outcomes is needed to optimize clinical benefit for all patients.
Keywords: cancer genomics; genomic tumor board; precision medicine; targeted therapeutics.
Conflict of interest statement
Alan Bryce has received honoraria, travel and accommodations from DAVA Oncology and had held a consulting/advisory role with Bayer. Steven Robinson has received travel and accommodations from Tracon. Aaron Mansfield has received honoraria from Celgene and Rockpointe and has held a consulting/advisory role with Genentech and Trovagene. Grzegorz Nowakowski has had a consulting/advisory role with Celgene, Bayer, Seattle Genetics, and Morphosis and research funding to institution from Celgene and Bayer and travel related to participation in investigator meeting from Celgene and Bayer. Robert McWilliams has held a consulting/advisory role with Merrimack and received funding from PRISM BioLab, Genentech, Novartis, Newlink Genetics, Lilly, Aduro Biotech, Pfizer, Sanofi and travel and accommodations from AstraZeneca.
Figures

References
-
- Tafe LJ, Gorlov IP, de Abreu FB, Lefferts JA, Liu X, Pettus JR, Marotti JD, Bloch KJ, Memoli VA, Suriawinata AA, Dragnev KH, Fadul CE, Schwartz GN, et al. Implementation of a Molecular Tumor Board: The Impact on Treatment Decisions for 35 Patients Evaluated at Dartmouth-Hitchcock Medical Center. Oncologist. 2015;20:1011–1018. - PMC - PubMed
-
- Sohal DP, Rini BI, Khorana AA, Dreicer R, Abraham J, Procop GW, Saunthararajah Y, Pennell NA, Stevenson JP, Pelley R, Estfan B, Shepard D, Funchain P. Prospective Clinical Study of Precision Oncology in Solid Tumors. J Natl Cancer Inst. 2015:108. - PubMed
-
- Schwaederle M, Parker BA, Schwab RB, Fanta PT, Boles SG, Daniels GA, Bazhenova LA, Subramanian R, Coutinho AC, Ojeda-Fournier H, Datnow B, Webster NJ, Lippman SM, et al. Molecular tumor board: the University of California-San Diego Moores Cancer Center experience. Oncologist. 2014;19:631–636. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

