Lenalidomide and the risk of serious infection in patients with multiple myeloma: a systematic review and meta-analysis
- PMID: 28423741
- PMCID: PMC5542295
- DOI: 10.18632/oncotarget.16235
Lenalidomide and the risk of serious infection in patients with multiple myeloma: a systematic review and meta-analysis
Abstract
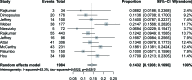
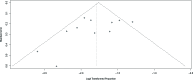
The immunomodulatory drug lenalidomide is highly effective against newly diagnosed and relapsed/refractory multiple myeloma (MM), but serious and even fatal infections have been associated with its use. In this meta-analysis, we assessed the overall risk of infection to MM patients treated with lenalidomide. Eleven phase II or III clinical trials, comprising 3,210 subjects, were selected from the Embase, Pubmed, and Cochrane Library databases, from the Clinical Trial Registration website, and from meeting abstracts and virtual presentations at the American Society of Clinical Oncology. Main outcome measures were overall incidence, relative risk (RR), and 95% confidence intervals (CIs) of reported infection events. Fixed-effect or random-effect models were used in the statistical analyses, depending on the between-study heterogeneity. The overall incidence of high-grade infection was 14.32% (95% CI: 12.08%-16.90%) and high-grade infection's pooled RR was 2.23 (95% CI: 1.71-2.91, P < 0.0001) for all 11 studies evaluated. No evidence of publication bias for the incidence of high-grade infection was detected using Begg's funnel plot and Egger's test (P = 0.2; 95% CI: -1.70, 1.23). From this meta-analysis, it appears lenalidomide use is associated with an increased risk of high-grade infection. Moreover, fatal infection events occurred only in patients treated with lenalidomide; no infection-related deaths were observed among controls. These data indicate that accurate diagnosis and optimal management of infection in MM patients treated with lenalidomide could be critical for treatment efficacy.
Keywords: incidence; infection; lenalidomide; meta-analysis; multiple myeloma.
Conflict of interest statement
There are no financial/commercial conflicts of interest involving any of the authors of this study.
Figures


References
-
- Hou J, Du X, Jin J, Cai Z, Chen F, Zhou DB, Yu L, Ke X, Li X, Wu D, Meng F, Ai H, Zhang J, et al. A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol. 2013;6:41. doi: 10.1186/1756-8722-6-41. - DOI - PMC - PubMed
-
- Zonder JA, Hussein MA, Bolejack V, Moore DF, Sr, Whittenberger BF, Abidi MH, Durie BGM, Barlogie B. Superiority of Lenalidomide (Len) Plus High-Dose Dexamethasone (HD) Compared to HD Alone as Treatment of Newly-Diagnosed Multiple Myeloma (NDMM): Results of the Randomized, Double-Blinded, Placebo-Controlled SWOG Trial S0232. Blood. 2007;110 abstract.
-
- Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, Zeldenrust SR, Kumar S, Greipp PR, Fonseca R, Lust JA, Russell SJ, Kyle RA, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–3. doi: 10.1182/blood-2005-07-2817. - DOI - PMC - PubMed
-
- Handa H, Saitoh T, Murakami H. [Immunomodulatory effects of lenalidomide]. [Article in Japanese] Nihon Rinsho. 2015;73:156–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

