Effectiveness of triple therapy with direct-acting antivirals for hepatitis C genotype 1 infection: application of propensity score matching in a national HCV treatment registry
- PMID: 28424064
- PMCID: PMC5395881
- DOI: 10.1186/s12913-017-2188-1
Effectiveness of triple therapy with direct-acting antivirals for hepatitis C genotype 1 infection: application of propensity score matching in a national HCV treatment registry
Abstract
Background: Observational studies are used to measure the effectiveness of an intervention in non-experimental, real world scenarios at the population level and are recognised as an important component of the evidence pyramid. Such data can be accrued through prospective cohort studies and a patient registry is a proven method for this type of study. The national hepatitis C (HCV) registry was established in Ireland in 2012 with the aim of monitoring the clinical and economic outcomes from new, high cost regimens for the treatment of HCV infection. A sustained virological response (SVR) 24 weeks following completion of therapy with interferon-containing regimens is considered a cure. Non-randomisation in these studies can result in confounding or selection bias. Propensity score (PS) matching is one of a number of statistical tools that can be used to mitigate the effects of confounding in observational studies.
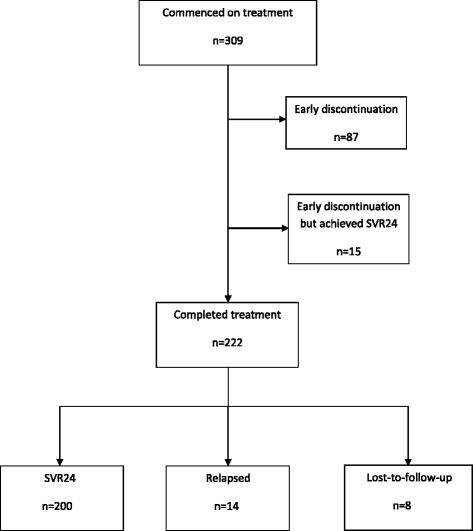
Methods: We analysed the data of 309 patients who underwent triple therapy treatment with telaprevir (TPV) in combination with pegylated-interferon and ribavirin (PR) or boceprevir (BOC)/PR between June 2012 and December 2014. The decision to initiate treatment and the selection of the treatment regimen was at the discretion of the physician. To adjust for confounding, three approaches to propensity score matching were assessed Adjusted sustained-virological response rates (SVR), odds ratios, p-values and 95% confidence intervals were calculated from the three PS matched dataset.
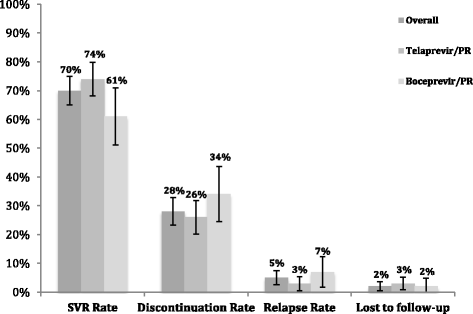
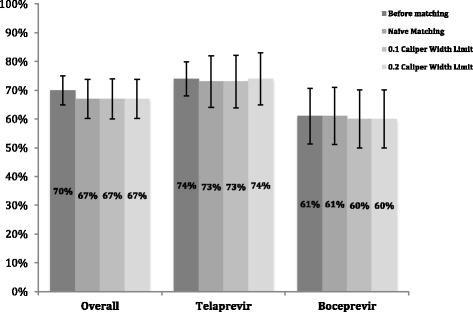
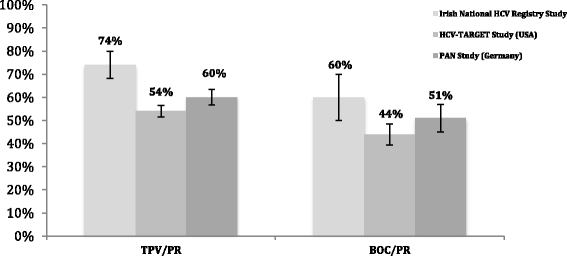
Results: Prior to matching, the unadjusted sustained virological response rates 24 weeks after treatment complete (SVR24) were 74% (n = 158/215) and 61% (n = 57/94) for telaprevir/PR and boceprevir/PR, respectively. After matching, adjusted SVR24 rates were between 73-74% and 60-61% for telaprevir/PR and boceprevir/PR, respectively.
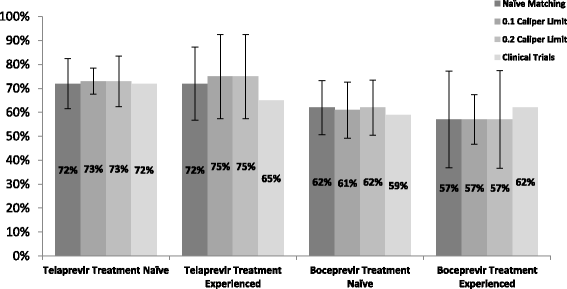
Conclusion: Efficacy rates were comparable with those reported in pivotal clinical trials and real world studies. After adjusting for confounding, we conclude that there was no difference in treatment effect after PS matching. The small sample size limits the conclusions that can be made about the effect of PS matching. Propensity score adjustment remains a tool that can be applied to future analysis, however, we suggest, where possible, using a larger sample size in order to reduce the uncertainty around the outcomes.
Keywords: Boceprevir; Comparative effectiveness; Outcomes Research; Propensity score matching; Protease inhibitor; Sustained virological response; Telaprevir.
Figures
Similar articles
-
Cost effectiveness of direct-acting antiviral therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration.Clin Gastroenterol Hepatol. 2013 Nov;11(11):1503-10. doi: 10.1016/j.cgh.2013.05.014. Epub 2013 May 22. Clin Gastroenterol Hepatol. 2013. PMID: 23707354
-
Cost-effectiveness of telaprevir in combination with pegylated interferon alpha and ribavirin in previously untreated chronic hepatitis C genotype 1 patients.J Med Econ. 2014 Jan;17(1):65-76. doi: 10.3111/13696998.2013.860033. Epub 2013 Nov 18. J Med Econ. 2014. PMID: 24160335
-
Cost-effectiveness of telaprevir in combination with pegylated interferon alpha and ribavirin in treatment-experienced chronic hepatitis C genotype 1 patients.J Med Econ. 2014 Jan;17(1):77-87. doi: 10.3111/13696998.2013.844159. Epub 2013 Nov 21. J Med Econ. 2014. PMID: 24032626
-
UK consensus guidelines for the use of the protease inhibitors boceprevir and telaprevir in genotype 1 chronic hepatitis C infected patients.Aliment Pharmacol Ther. 2012 Mar;35(6):647-62. doi: 10.1111/j.1365-2036.2012.04992.x. Epub 2012 Feb 1. Aliment Pharmacol Ther. 2012. PMID: 22296568
-
Impact of HCV protease-inhibitor-based triple therapy for chronic HCV genotype 1 infection.Antivir Ther. 2011;16(8):1187-201. doi: 10.3851/IMP1934. Antivir Ther. 2011. PMID: 22155901 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

