Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury
- PMID: 28424078
- PMCID: PMC5397701
- DOI: 10.1186/s12931-017-0553-6
Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury
Abstract
Background: Interleukin 6 (IL-6) is a predictive factor of poor prognosis in patients with acute respiratory distress syndrome (ARDS). However, its acute pulmonary hemodynamic effects and role in lung injury have not been investigated in a clinically relevant murine model of ARDS.
Methods: We used adult C57Bl6 wild-type (WT) and IL-6 knock-out (IL-6KO) mice. The animals received intravenous recombinant human IL-6 (rHuIL-6) or vehicle followed by intratracheal lipopolysaccharide (LPS) or saline before undergoing low tidal volume mechanical ventilation (MV) for 5 h. Before sacrifice, right ventricular systolic pressure and cardiac output were measured and total pulmonary resistance was calculated. After sacrifice, lung inflammation, edema and injury were assessed with bronchoalveolar lavage (BAL) and histology. In other experiments, right ventricular systolic pressure was recorded during hypoxic challenges in uninjured WT mice pretreated with rHuIL-6 or rHuIL-6 + non-selective nitric oxide synthase inhibitor L-NAME or vehicle.
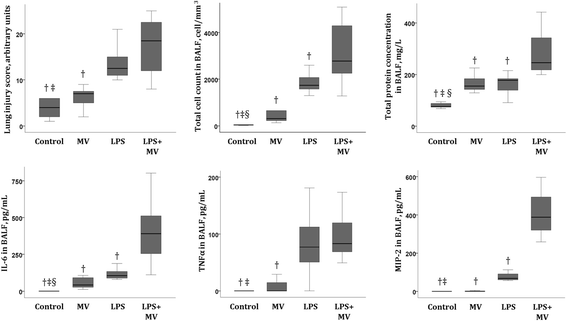
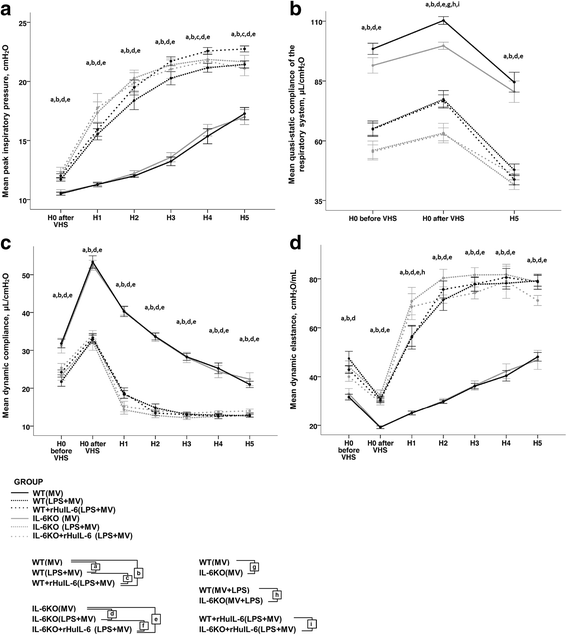
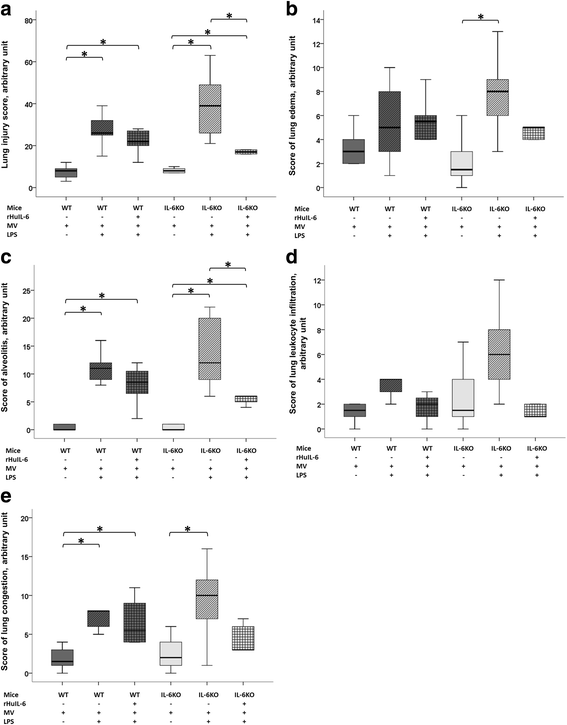
Results: IL-6KO(LPS+MV) mice showed a faster deterioration of lung elastic properties and more severe bronchoalveolar cellular inflammation as compared to WT(LPS+MV). Treatment with rHuIL-6 partially prevented this lung deterioration. Total pulmonary resistance was higher in IL-6KO(LPS+MV) mice and this increase was abolished in rHuIL-6-treated IL-6KO mice. Finally, rHuIL-6 reduced hypoxic pulmonary vasoconstriction in uninjured WT mice, an effect that was abolished by co-treatment with L-NAME.
Conclusions: In a double-hit murine model of ARDS, IL-6 deficient mice experienced more severe bronchoalveolar cellular inflammation as compared to wild-type littermates. Furthermore, IL-6 deficiency caused marked acute pulmonary hypertension, which may be, at least partially, due to vasoactive mechanisms. A dysregulation of nitric oxide synthase may account for this observation, a hypothesis that will need to be investigated in future studies.
Keywords: Acute lung injury; Acute respiratory distress syndrome; Interleukin-6; Mechanical ventilation; Nitric oxide synthase; Pulmonary hypertension.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

