Clinical significance of somatic mutation in unexplained blood cytopenia
- PMID: 28424163
- PMCID: PMC5542849
- DOI: 10.1182/blood-2017-01-763425
Clinical significance of somatic mutation in unexplained blood cytopenia
Abstract
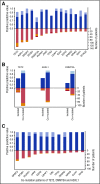
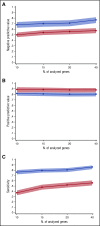
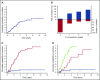
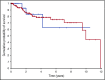
Unexplained blood cytopenias, in particular anemia, are often found in older persons. The relationship between these cytopenias and myeloid neoplasms like myelodysplastic syndromes is currently poorly defined. We studied a prospective cohort of patients with unexplained cytopenia with the aim to estimate the predictive value of somatic mutations for identifying subjects with, or at risk of, developing a myeloid neoplasm. The study included a learning cohort of 683 consecutive patients investigated for unexplained cytopenia, and a validation cohort of 190 patients referred for suspected myeloid neoplasm. Using granulocyte DNA, we looked for somatic mutations in 40 genes that are recurrently mutated in myeloid malignancies. Overall, 435/683 patients carried a somatic mutation in at least 1 of these genes. Carrying a somatic mutation with a variant allele frequency ≥0.10, or carrying 2 or more mutations, had a positive predictive value for diagnosis of myeloid neoplasm equal to 0.86 and 0.88, respectively. Spliceosome gene mutations and comutation patterns involving TET2, DNMT3A, or ASXL1 had positive predictive values for myeloid neoplasm ranging from 0.86 to 1.0. Within subjects with inconclusive diagnostic findings, carrying 1 or more somatic mutations was associated with a high probability of developing a myeloid neoplasm during follow-up (hazard ratio = 13.9, P < .001). The predictive values of mutation analysis were confirmed in the independent validation cohort. The findings of this study indicate that mutation analysis on peripheral blood granulocytes may significantly improve the current diagnostic approach to unexplained cytopenia and more generally the diagnostic accuracy of myeloid neoplasms.
© 2017 by The American Society of Hematology.
Figures

Comment in
-
Now I cuss less about ICUS.Blood. 2017 Jun 22;129(25):3282-3283. doi: 10.1182/blood-2017-05-781245. Blood. 2017. PMID: 28642360 No abstract available.
References
-
- Vanasse GJ, Berliner N. Anemia in elderly patients: an emerging problem for the 21st century. Hematology Am Soc Hematol Educ Program. 2010;2010:271-275. - PubMed
-
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

