The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness
- PMID: 28424209
- PMCID: PMC5625101
- DOI: 10.1152/ajprenal.00096.2017
The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness
Abstract
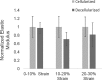
Basement membranes (BMs), a specialized form of extracellular matrix, underlie nearly all cell layers and provide structural support for tissues and interact with cell surface receptors to determine cell behavior. Both macromolecular composition and stiffness of the BM influence cell-BM interactions. Collagen IV is a major constituent of the BM that forms an extensively cross-linked oligomeric network. Its deficiency leads to BM mechanical instability, as observed with glomerular BM in Alport syndrome. These findings have led to the hypothesis that collagen IV and its cross-links determine BM stiffness. A sulfilimine bond (S = N) between a methionine sulfur and a lysine nitrogen cross-links collagen IV and is formed by the matrix enzyme peroxidasin. In peroxidasin knockout mice with reduced collagen IV sulfilimine cross-links, we find a reduction in renal tubular BM stiffness. Thus this work provides the first direct experimental evidence that collagen IV sulfilimine cross-links contribute to BM mechanical properties and provides a foundation for future work on the relationship of BM mechanics to cell function in renal disease.
Keywords: basement membrane; collagen IV; elastic modulus; peroxidasin; sulfilimine cross-link.
Copyright © 2017 the American Physiological Society.
Figures





Similar articles
-
Peroxidasin-mediated bromine enrichment of basement membranes.Proc Natl Acad Sci U S A. 2020 Jul 7;117(27):15827-15836. doi: 10.1073/pnas.2007749117. Epub 2020 Jun 22. Proc Natl Acad Sci U S A. 2020. PMID: 32571911 Free PMC article.
-
The Ancient Immunoglobulin Domains of Peroxidasin Are Required to Form Sulfilimine Cross-links in Collagen IV.J Biol Chem. 2015 Aug 28;290(35):21741-8. doi: 10.1074/jbc.M115.673996. Epub 2015 Jul 15. J Biol Chem. 2015. PMID: 26178375 Free PMC article.
-
A unique covalent bond in basement membrane is a primordial innovation for tissue evolution.Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):331-6. doi: 10.1073/pnas.1318499111. Epub 2013 Dec 16. Proc Natl Acad Sci U S A. 2014. PMID: 24344311 Free PMC article.
-
Role of Hypohalous Acids in Basement Membrane Homeostasis.Antioxid Redox Signal. 2017 Oct 20;27(12):839-854. doi: 10.1089/ars.2017.7245. Epub 2017 Jul 31. Antioxid Redox Signal. 2017. PMID: 28657332 Free PMC article. Review.
-
Sulfilimine bond formation in collagen IV.Chem Commun (Camb). 2024 Jan 16;60(6):646-657. doi: 10.1039/d3cc05715a. Chem Commun (Camb). 2024. PMID: 38116662 Review.
Cited by
-
Peroxidasin-mediated bromine enrichment of basement membranes.Proc Natl Acad Sci U S A. 2020 Jul 7;117(27):15827-15836. doi: 10.1073/pnas.2007749117. Epub 2020 Jun 22. Proc Natl Acad Sci U S A. 2020. PMID: 32571911 Free PMC article.
-
Biallelic Deletion of Pxdn in Mice Leads to Anophthalmia and Severe Eye Malformation.Int J Mol Sci. 2019 Dec 5;20(24):6144. doi: 10.3390/ijms20246144. Int J Mol Sci. 2019. PMID: 31817535 Free PMC article.
-
Renal Biology Driven Macro- and Microscale Design Strategies for Creating an Artificial Proximal Tubule Using Fiber-Based Technologies.ACS Biomater Sci Eng. 2021 Oct 11;7(10):4679-4693. doi: 10.1021/acsbiomaterials.1c00408. Epub 2021 Sep 7. ACS Biomater Sci Eng. 2021. PMID: 34490771 Free PMC article. Review.
-
Collagen type IV as the link between arterial stiffness and dementia.Am J Transl Res. 2023 Oct 15;15(10):5961-5971. eCollection 2023. Am J Transl Res. 2023. PMID: 37969177 Free PMC article. Review.
-
Aortic Stiffness in L-NAME Treated C57Bl/6 Mice Displays a Shift From Early Endothelial Dysfunction to Late-Term Vascular Smooth Muscle Cell Dysfunction.Front Physiol. 2022 Jun 16;13:874015. doi: 10.3389/fphys.2022.874015. eCollection 2022. Front Physiol. 2022. PMID: 35800344 Free PMC article.
References
-
- Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, Kang JS, Pedchenko V, Fessler LI, Fessler JH, Hudson BG. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8: 784–790, 2012. doi:10.1038/nchembio.1038. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

