Low-Dose Paclitaxel-Coated Versus Uncoated Percutaneous Transluminal Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease: One-Year Results of the ILLUMENATE European Randomized Clinical Trial (Randomized Trial of a Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon)
- PMID: 28424223
- PMCID: PMC5459585
- DOI: 10.1161/CIRCULATIONAHA.116.026493
Low-Dose Paclitaxel-Coated Versus Uncoated Percutaneous Transluminal Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease: One-Year Results of the ILLUMENATE European Randomized Clinical Trial (Randomized Trial of a Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon)
Abstract
Background: Numerous studies have reported favorable outcomes using drug-coated balloons (DCBs) for treatment of symptomatic peripheral artery disease of the superficial femoral and popliteal arteries. However, the treatment effect compared with an uncoated balloon has differed greatly among the randomized trials, with better outcomes observed with higher-dose DCBs. This European trial was designed to assess the safety and effectiveness of a next-generation low-dose (2-µg/mm2 surface dose of paclitaxel) DCB.
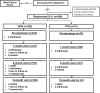
Methods: This was a prospective, randomized, multicenter, single-blinded trial. Patients were randomized (3:1) to treatment with a low-dose DCB or an uncoated percutaneous transluminal angioplasty (PTA) balloon. The primary safety end point was a composite of freedom from device- and procedure-related death through 30 days after the procedure and freedom from target limb major amputation and clinically driven target lesion revascularization through 12 months after the procedure. The primary effectiveness end point was primary patency at 12 months.
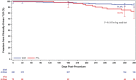
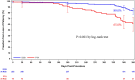
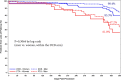
Results: Patients were randomized to treatment with a DCB (222 patients, 254 lesions) or uncoated PTA balloon (72 patients, 79 lesions) after successful predilatation. Mean lesion length was 7.2 and 7.1 cm, and 19.2% and 19.0% of lesions represented total occlusions, respectively. The primary safety end point was met, and superiority was demonstrated; freedom from a primary safety event was 94.1% (193 of 205) with DCB and 83.3% (50 of 60) with PTA, for a difference of 10.8% (95% confidence interval, 0.9%-23.0%). The primary effectiveness end point was met, and superiority of DCB over PTA was achieved (83.9% [188 of 224] versus 60.6% [40 of 66]; P<0.001). Outcomes with DCB were also superior to PTA per the Kaplan-Meier estimate for primary patency (89.0% versus 65.0% at 365 days; log-rank P<0.001) and for rates of clinically driven target lesion revascularization (5.9% versus 16.7%; P=0.014).
Conclusions: Superiority with a low-dose DCB for femoropopliteal interventions was demonstrated over PTA for both the safety and effectiveness end points.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01858363.
Keywords: drug-eluting balloon; paclitaxel; percutaneous treatment; peripheral artery disease; randomized controlled trial.
© 2017 The Authors.
Figures

Comment in
-
Novel Strategies to Reduce Femoropopliteal Restenosis: Low-Dose Paclitaxel-Coated Balloons and Paclitaxel-Coated Balloons Plus Stenting.Circulation. 2017 Jun 6;135(23):2237-2240. doi: 10.1161/CIRCULATIONAHA.117.028308. Epub 2017 Apr 19. Circulation. 2017. PMID: 28424224 No abstract available.
References
-
- Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR, 3rd, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163:884–892. doi: 10.1001/archinte.163.8.884. - PubMed
-
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. - PubMed
-
- Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Müller-Hülsbeck S, Nehler MR, Benenati JF, Scheinert D LEVANT 2 Investigators. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. doi: 10.1056/NEJMoa1406235. - PubMed
-
- Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. - PMC - PubMed
-
- Vardi M, Novack V, Pencina MJ, Doros G, Burke DA, Elmariah S, Cutlip DE, Mauri L, Yeh RW. Safety and efficacy metrics for primary nitinol stenting in femoropopliteal occlusive disease: a meta-analysis and critical examination of current methodologies. Catheter Cardiovasc Interv. 2014;83:975–983. doi: 10.1002/ccd.25179. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

