Certainty of genuine treatment increases drug responses among intellectually disabled patients
- PMID: 28424273
- PMCID: PMC5444309
- DOI: 10.1212/WNL.0000000000003934
Certainty of genuine treatment increases drug responses among intellectually disabled patients
Abstract
Objective: To determine the placebo component of treatment responses in patients with intellectual disability (ID).
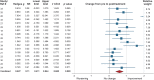
Methods: A statistical meta-analysis comparing bias-corrected effect sizes (Hedges g) of drug responses in open-label vs placebo-controlled clinical trials was performed, as these trial types represent different certainty of receiving genuine treatment (100% vs 50%). Studies in fragile X, Down, Prader-Willi, and Williams syndrome published before June 2015 were considered.
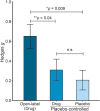
Results: Seventeen open-label trials (n = 261, 65% male; mean age 23.6 years; mean trial duration 38 weeks) and 22 placebo-controlled trials (n = 721, 62% male; mean age 17.1 years; mean trial duration 35 weeks) were included. The overall effect size from pre to post treatment in open-label studies was g = 0.602 (p = 0.001). The effect of trial type was statistically significant (p = 0.001), and revealed higher effect sizes in studies with 100% likelihood of getting active drug, compared to both the drug and placebo arm of placebo-controlled trials. We thus provide evidence for genuine placebo effects, not explainable by natural history or regression toward the mean, among patients with ID.
Conclusions: Our data suggest that clinical trials in patients with severe cognitive deficits are influenced by the certainty of receiving genuine medication, and open-label design should thus not be used to evaluate the effect of pharmacologic treatments in ID, as the results will be biased by an enhanced placebo component.
Copyright © 2017 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Similar articles
-
Placebo Responses in Genetically Determined Intellectual Disability: A Meta-Analysis.PLoS One. 2015 Jul 30;10(7):e0133316. doi: 10.1371/journal.pone.0133316. eCollection 2015. PLoS One. 2015. PMID: 26226597 Free PMC article.
-
Does Certainty of Genuine Treatment Increase the Drug Response in Alzheimer's Disease Patients: A Meta-Analysis and Critical Discussion.J Alzheimers Dis. 2021;84(4):1821-1832. doi: 10.3233/JAD-210108. J Alzheimers Dis. 2021. PMID: 34744076
-
Beginning reading interventions for children and adolescents with intellectual disability.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011359. doi: 10.1002/14651858.CD011359.pub2. Cochrane Database Syst Rev. 2019. PMID: 31805208 Free PMC article.
-
A Novel Analog Reasoning Paradigm: New Insights in Intellectually Disabled Patients.PLoS One. 2016 Feb 26;11(2):e0149717. doi: 10.1371/journal.pone.0149717. eCollection 2016. PLoS One. 2016. PMID: 26918704 Free PMC article.
-
Adjuvant therapy with antidepressants for the management of inflammatory bowel disease.Cochrane Database Syst Rev. 2019 Apr 12;4(4):CD012680. doi: 10.1002/14651858.CD012680.pub2. Cochrane Database Syst Rev. 2019. PMID: 30977111 Free PMC article.
Cited by
-
Why we need more research into the placebo response in psychiatry.Psychol Med. 2020 Oct;50(14):2317-2323. doi: 10.1017/S0033291720003633. Epub 2020 Oct 8. Psychol Med. 2020. PMID: 33028433 Free PMC article. Review.
-
Improving Public Health Requires Inclusion of Underrepresented Populations in Research.JAMA. 2018 Jan 23;319(4):337-338. doi: 10.1001/jama.2017.19138. JAMA. 2018. PMID: 29285540 Free PMC article. No abstract available.
-
Placebo and nocebo effects and mechanisms associated with pharmacological interventions: an umbrella review.BMJ Open. 2023 Oct 17;13(10):e077243. doi: 10.1136/bmjopen-2023-077243. BMJ Open. 2023. PMID: 37848293 Free PMC article.
-
Control interventions in randomised trials among people with mental health disorders.Cochrane Database Syst Rev. 2022 Apr 4;4(4):MR000050. doi: 10.1002/14651858.MR000050.pub2. Cochrane Database Syst Rev. 2022. PMID: 35377466 Free PMC article.
-
Mouse models of fragile X-related disorders.Dis Model Mech. 2023 Feb 1;16(2):dmm049485. doi: 10.1242/dmm.049485. Epub 2023 Jan 24. Dis Model Mech. 2023. PMID: 36692473 Free PMC article. Review.
References
-
- Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol 2004;3:679–684. - PubMed
-
- American Psychiatric Association. Intellectual Disability, 5th ed. Washington, DC: American Psychiatric Association; 2013.
-
- Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 2009;19:34–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
