Dosing Three-Drug Combinations That Include Targeted Anti-Cancer Agents: Analysis of 37,763 Patients
- PMID: 28424323
- PMCID: PMC5423521
- DOI: 10.1634/theoncologist.2016-0357
Dosing Three-Drug Combinations That Include Targeted Anti-Cancer Agents: Analysis of 37,763 Patients
Abstract
Background: Combining targeted and cytotoxic agents has the potential to improve efficacy and attenuate resistance for metastatic cancer. Information regarding safe starting doses for clinical trials of novel three-drug combinations is lacking.
Materials and methods: Published phase I-III adult oncology clinical trials of three-drug combinations involving a targeted agent were identified by PubMed search (January 1, 2010 to December 31, 2013). A dose percentage was calculated to compare the dose used in combination to the single agent recommended dose: (U.S. Food and Drug Administration-approved/recommended phase II dose/maximum tolerated dose). The additive dose percentage was the sum of the dose percentages for each drug in the combination.
Results: A total of 37,763 subjects and 243 drug combinations were included. Only 28% of studies could give each of the three agents at 100%. For combinations involving two targeted agents and a cytotoxic agent, the lowest starting additive dose percentage was 133%, which increased to 250% if two antibodies were included. For combinations of one targeted agent and two cytotoxic agents, the lowest additive safe dose percentage was 137%. When both cytotoxic agents were held at 100%, as occurred in 56% of studies (which generally used cytotoxic doublets with known combination safety dosing), the lowest safe dose percentage was 225% (providing that a histone deacetylase inhibitor was not the targeted agent).
Conclusion: These findings serve as a safe starting point for dosing novel three-drug combinations involving a targeted agent in clinical trials and practice. The Oncologist 2017;22:576-584 IMPLICATIONS FOR PRACTICE: Targeted and cytotoxic drug combinations can improve efficacy and overcome resistance. More knowledge of safe starting doses would facilitate use of combinations in clinical trials and practice. Analysis of 37,763 subjects (243 combinations) showed three drugs could be safely administered, but less than 30% of combinations could include all three drugs at full dose. Dose reductions to 45% of the dose of each single agent may be required. Combinations involving two antibodies required fewer dose reductions, and the use of established cytotoxic doublets made initial dose assignment easier.
Keywords: Cytotoxic chemotherapy; Maximum tolerated dose; Precision medicine; Recommended phase II dose; Targeted therapy.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
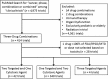
References
-
- Averbuch SD, Wolf MK, El‐Rayes BF et al. Clinical development issues. In: Prendergast GC, ed. Molecular Cancer Therapeutics: Strategies for Drug Discovery and Development. Hoboken, NJ: Wiley‐LISS, 2004:287–306.
-
- Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Eng J Med 2004;350:2335–2342. - PubMed
-
- Sehn LH, Donaldson J, Chhanabhai M et al. Introduction of combined chop plus rituximab therapy dramatically improved outcome of diffuse large b‐cell lymphoma in British Columbia. J Clin Oncol 2005;23:5027–5033. - PubMed
-
- Baselga J. Herceptin alone or in combination with chemotherapy in the treatment of HER2‐positive metastatic breast cancer: Pivotal trials. Oncology 2001;61(suppl 2):14–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

