RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury
- PMID: 28424327
- PMCID: PMC5604790
- DOI: 10.1126/scitranslmed.aag2513
RIG-I/MAVS and STING signaling promote gut integrity during irradiation- and immune-mediated tissue injury
Abstract
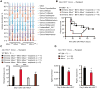
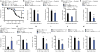
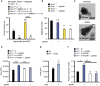
The molecular pathways that regulate the tissue repair function of type I interferon (IFN-I) during acute tissue damage are poorly understood. We describe a protective role for IFN-I and the RIG-I/MAVS signaling pathway during acute tissue damage in mice. Mice lacking mitochondrial antiviral-signaling protein (MAVS) were more sensitive to total body irradiation- and chemotherapy-induced intestinal barrier damage. These mice developed worse graft-versus-host disease (GVHD) in a preclinical model of allogeneic hematopoietic stem cell transplantation (allo-HSCT) than did wild-type mice. This phenotype was not associated with changes in the intestinal microbiota but was associated with reduced gut epithelial integrity. Conversely, targeted activation of the RIG-I pathway during tissue injury promoted gut barrier integrity and reduced GVHD. Recombinant IFN-I or IFN-I expression induced by RIG-I promoted growth of intestinal organoids in vitro and production of the antimicrobial peptide regenerating islet-derived protein 3 γ (RegIIIγ). Our findings were not confined to RIG-I/MAVS signaling because targeted engagement of the STING (stimulator of interferon genes) pathway also protected gut barrier function and reduced GVHD. Consistent with this, STING-deficient mice suffered worse GVHD after allo-HSCT than did wild-type mice. Overall, our data suggest that activation of either RIG-I/MAVS or STING pathways during acute intestinal tissue injury in mice resulted in IFN-I signaling that maintained gut epithelial barrier integrity and reduced GVHD severity. Targeting these pathways may help to prevent acute intestinal injury and GVHD during allogeneic transplantation.
Copyright © 2017, American Association for the Advancement of Science.
Figures




References
-
- Abdullah Z, Schlee M, Roth S, Mraheil MA, Barchet W, Böttcher J, Hain T, Geiger S, Hayakawa Y, Fritz JH, Civril F, Hopfner KP, Kurts C, Ruland J, Hartmann G, Chakraborty T, Knolle PA. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J. 2012;31:4153–4164. - PMC - PubMed
-
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. - PubMed
-
- Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. - PubMed
-
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2014;35:88–93. - PubMed
-
- Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschläger N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1b production. Nat Immunol. 2010;11:63–69. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

