Tau haploinsufficiency causes prenatal loss of dopaminergic neurons in the ventral tegmental area and reduction of transcription factor orthodenticle homeobox 2 expression
- PMID: 28424350
- PMCID: PMC5503702
- DOI: 10.1096/fj.201601303R
Tau haploinsufficiency causes prenatal loss of dopaminergic neurons in the ventral tegmental area and reduction of transcription factor orthodenticle homeobox 2 expression
Abstract
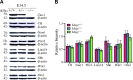
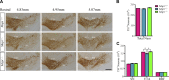
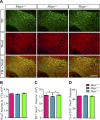
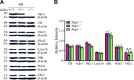
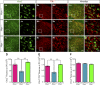
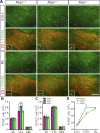
Homozygous tau knockout (Mapt-/-) mice develop age-dependent dopaminergic (DA) neuronal loss in the substantia nigra (SN) and ventral tegmental area (VTA), supporting an important function of tau in maintaining the survival of midbrain dopaminergic neurons (mDANs) during aging. However, it remains to be determined whether the microtubule-associated protein tau regulates the differentiation and survival of mDANs during embryonic developmental stages. Here, we show that tau haploinsufficiency in postnatal day 0 (P0) heterozygous (Mapt+/-) pups, but not a complete loss of tau in the Mapt-/- littermates, led to a significant reduction of DA neurons in the VTA. This selective loss of DA neurons correlated with a similar reduction in orthodenticle homeobox 2 (Otx2), which is restricted to VTA neurons at the postmitotic stage and selectively controls the neurogenesis and survival of specific neuronal subtypes of VTA. Moreover, the prenatal developmental cell death in the Mapt+/- VTA specifically increased, and the expression of microtubule-associated protein (MAP)-1A was significantly up-regulated in the P0 Mapt-/- , but not the Mapt+/- , pups. These results suggest that tau haploinsufficiency, without the compensation effect of MAP1A, induces reduction of Otx2 expression, increases prenatal cell death, and accordingly leads to selective loss of VTA DA neurons in the early postnatal stage. Our findings highlight the impact of tau haploinsufficiency on the survival of mDANs and indicate that tau may participate in midbrain development in a dose-dependent way.-Zheng, M., Jiao, L., Tang, X., Xiang, X., Wan, X., Yan, Y., Li, X., Zhang, G., Li, Y., Jiang, B., Cai, H., Lin, X. Tau haploinsufficiency causes prenatal loss of dopaminergic neurons in the ventral tegmental area and reduction of transcription factor orthodenticle homeobox 2 expression.
Keywords: MAP1A; compensation effect; midbrain development; tau deficiency.
© FASEB.
Figures


Similar articles
-
Tau Deficiency Down-Regulated Transcription Factor Orthodenticle Homeobox 2 Expression in the Dopaminergic Neurons in Ventral Tegmental Area and Caused No Obvious Motor Deficits in Mice.Neuroscience. 2018 Mar 1;373:52-59. doi: 10.1016/j.neuroscience.2018.01.002. Epub 2018 Jan 11. Neuroscience. 2018. PMID: 29337233 Free PMC article.
-
Otx2 selectively controls the neurogenesis of specific neuronal subtypes of the ventral tegmental area and compensates En1-dependent neuronal loss and MPTP vulnerability.Dev Biol. 2013 Jan 1;373(1):176-83. doi: 10.1016/j.ydbio.2012.10.022. Epub 2012 Oct 29. Dev Biol. 2013. PMID: 23117062
-
Otx2 expression is restricted to dopaminergic neurons of the ventral tegmental area in the adult brain.Int J Dev Biol. 2010;54(5):939-45. doi: 10.1387/ijdb.092974ms. Int J Dev Biol. 2010. PMID: 19924631
-
The role of otx2 in adult mesencephalic-diencephalic dopaminergic neurons.Mol Neurobiol. 2011 Apr;43(2):107-13. doi: 10.1007/s12035-010-8148-y. Epub 2010 Nov 18. Mol Neurobiol. 2011. PMID: 21086067 Review.
-
Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat: Historical perspective.Brain Res. 2016 Aug 15;1645:68-70. doi: 10.1016/j.brainres.2015.12.041. Epub 2015 Dec 28. Brain Res. 2016. PMID: 26731335 Review.
Cited by
-
Much More Than a Cytoskeletal Protein: Physiological and Pathological Functions of the Non-microtubule Binding Region of Tau.Front Neurol. 2020 Oct 19;11:590059. doi: 10.3389/fneur.2020.590059. eCollection 2020. Front Neurol. 2020. PMID: 33193056 Free PMC article. Review.
-
The loss of dopaminergic neurons in DEC1 deficient mice potentially involves the decrease of PI3K/Akt/GSK3β signaling.Aging (Albany NY). 2019 Dec 28;11(24):12733-12753. doi: 10.18632/aging.102599. Epub 2019 Dec 28. Aging (Albany NY). 2019. PMID: 31884423 Free PMC article.
-
Tau knockout exacerbates degeneration of parvalbumin-positive neurons in substantia nigra pars reticulata in Parkinson's disease-related α-synuclein A53T mice.FASEB J. 2020 Sep;34(9):12239-12254. doi: 10.1096/fj.202000017RR. Epub 2020 Jul 30. FASEB J. 2020. PMID: 33000527 Free PMC article.
-
The six brain-specific TAU isoforms and their role in Alzheimer's disease and related neurodegenerative dementia syndromes.Alzheimers Dement. 2024 May;20(5):3606-3628. doi: 10.1002/alz.13784. Epub 2024 Mar 31. Alzheimers Dement. 2024. PMID: 38556838 Free PMC article. Review.
-
Roles of Transcription Factors in the Development and Reprogramming of the Dopaminergic Neurons.Int J Mol Sci. 2022 Jan 13;23(2):845. doi: 10.3390/ijms23020845. Int J Mol Sci. 2022. PMID: 35055043 Free PMC article. Review.
References
-
- Johnson G. V., Stoothoff W. H. (2004) Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 117, 5721–5729 - PubMed
-
- Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 - PubMed
-
- Lei P., Ayton S., Finkelstein D. I., Adlard P. A., Masters C. L., Bush A. I. (2010) Tau protein: relevance to Parkinson’s disease. Int. J. Biochem. Cell Biol. 42, 1775–1778 - PubMed
-
- Schapira A. H. (1997) Pathogenesis of Parkinson’s disease. Baillieres Clin. Neurol. 6, 15–36 - PubMed
-
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Alpha-synuclein in Lewy bodies. Nature 388, 839–840 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

