Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD
- PMID: 28424359
- PMCID: PMC5898947
- DOI: 10.1183/13993003.01348-2016
Effects of combined tiotropium/olodaterol on inspiratory capacity and exercise endurance in COPD
Abstract
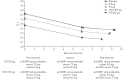
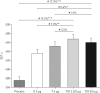
Two replicate, double-blind, 6-week, incomplete-crossover studies (MORACTO 1 and 2) assessed the effects of tiotropium/olodaterol on inspiratory capacity and exercise endurance time in patients with moderate to severe chronic obstructive pulmonary disease.For each patient, four of five treatments were administered once daily for 6 weeks, with a 21-day washout between treatments: tiotropium/olodaterol 2.5/5 µg or 5/5 µg, tiotropium 5 µg, olodaterol 5 µg or placebo, all via the Respimat inhaler. Primary outcomes were inspiratory capacity prior to exercise and exercise endurance time during constant work-rate cycle ergometry to symptom limitation at 75% of peak incremental work rate after 6 weeks (2 h post-dose).295 and 291 patients were treated in MORACTO 1 and 2, respectively. Tiotropium/olodaterol 2.5/5 and 5/5 µg provided significant improvements in inspiratory capacity versus placebo and monotherapies (p<0.0001), and significant improvements in exercise endurance time versus placebo (p<0.0001). Intensity of breathing discomfort was reduced following both doses of tiotropium/olodaterol versus placebo (p<0.0001).Once-daily tiotropium/olodaterol yielded improvements in lung hyperinflation versus placebo and statistically significant improvements versus monotherapies. Tiotropium/olodaterol also showed improvements in dyspnoea and exercise tolerance versus placebo but not consistently versus monotherapies.
Trial registration: ClinicalTrials.gov NCT01533935 NCT01533922.
Copyright ©ERS 2017.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside this article at erj.ersjournals.com
Figures


Comment in
-
Chronic obstructive pulmonary disease: inhale deeply and start to exercise.Eur Respir J. 2017 Apr 19;49(4):1700424. doi: 10.1183/13993003.00424-2017. Print 2017 Apr. Eur Respir J. 2017. PMID: 28424367 No abstract available.
References
-
- ZuWallack R. How are you doing? What are you doing? Differing perspectives in the assessment of individuals with COPD. COPD 2007; 4: 293–297. - PubMed
-
- Jones PW. Activity limitation and quality of life in COPD. COPD 2007; 4: 273–278. - PubMed
-
- O'Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD 2007; 4: 225–236. - PubMed
-
- Garcia-Rio F, Lores V, Mediano O, et al. . Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med 2009; 180: 506–512. - PubMed
-
- Watz H, Pitta F, Rochester CL, et al. . An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44: 1521–1537. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
