Azathioprine, Mercaptopurine, and 5-Aminosalicylic Acid Affect the Growth of IBD-Associated Campylobacter Species and Other Enteric Microbes
- PMID: 28424670
- PMCID: PMC5372805
- DOI: 10.3389/fmicb.2017.00527
Azathioprine, Mercaptopurine, and 5-Aminosalicylic Acid Affect the Growth of IBD-Associated Campylobacter Species and Other Enteric Microbes
Abstract
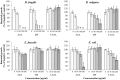
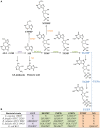
Campylobacter concisus is a bacterium that is associated with inflammatory bowel disease (IBD). Immunosuppressive drugs including azathioprine (AZA) and mercaptopurine (MP), and anti-inflammatory drug such as 5-aminosalicylic acid (5-ASA) are commonly used to treat patients with IBD. This study aimed to examine the effects of AZA, MP, and 5-ASA on the growth of IBD-associated bacterial species and to identify bacterial enzymes involved in immunosuppressive drug metabolism. A total of 15 bacterial strains of five species including 11 C. concisus strains, Bacteroides fragilis, Bacteroides vulgatus, Enterococcus faecalis, and Escherichia coli were examined. The impact of AZA, MP, and 5-ASA on the growth of these bacterial species was examined quantitatively using a plate counting method. The presence of enzymes involved in AZA and MP metabolism in these bacterial species was identified using bioinformatics tools. AZA and MP significantly inhibited the growth of all 11 C. concisus strains. C. concisus strains were more sensitive to AZA than MP. 5-ASA showed inhibitory effects to some C. concisus strains, while it promoted the growth of other C. concisus strains. AZA and MP also significantly inhibited the growth of B. fragilis and B. vulgatus. The growth of E. coli was significantly inhibited by 200 μg/ml of AZA as well as 100 and 200 μg/ml of 5-ASA. Bacterial enzymes related to AZA and MP metabolism were found, which varied in different bacterial species. In conclusion, AZA and MP have inhibitory effects to IBD-associated C. concisus and other enteric microbes, suggesting an additional therapeutic mechanism of these drugs in the treatment of IBD. The strain dependent differential impact of 5-ASA on the growth of C. concisus may also have clinical implication given that in some cases 5-ASA medications were found to cause exacerbations of colitis.
Keywords: 5-aminosalicylic acid; Campylobacter concisus; anti-inflammatory drug; azathioprine; enteric bacteria; immunosuppressive drug; inflammatory bowel disease; mercaptopurine.
Figures


Similar articles
-
Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease.PLoS One. 2012;7(5):e38217. doi: 10.1371/journal.pone.0038217. Epub 2012 May 30. PLoS One. 2012. PMID: 22666490 Free PMC article.
-
5-aminosalicylate therapy is associated with higher 6-thioguanine levels in adults and children with inflammatory bowel disease in remission on 6-mercaptopurine or azathioprine.Inflamm Bowel Dis. 2006 Apr;12(4):251-7. doi: 10.1097/01.MIB.0000206544.05661.9f. Inflamm Bowel Dis. 2006. PMID: 16633046
-
Delineation of genetic relatedness and population structure of oral and enteric Campylobacter concisus strains by analysis of housekeeping genes.Microbiology (Reading). 2015 Aug;161(8):1600-1612. doi: 10.1099/mic.0.000112. Epub 2015 May 22. Microbiology (Reading). 2015. PMID: 26002953
-
Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review.World J Gastroenterol. 2019 Mar 7;25(9):1142-1157. doi: 10.3748/wjg.v25.i9.1142. World J Gastroenterol. 2019. PMID: 30863001 Free PMC article.
-
The Clinical Importance of Campylobacter concisus and Other Human Hosted Campylobacter Species.Front Cell Infect Microbiol. 2018 Jul 24;8:243. doi: 10.3389/fcimb.2018.00243. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30087857 Free PMC article. Review.
Cited by
-
The Growth and Protein Expression of Inflammatory Bowel Disease-Associated Campylobacter concisus Is Affected by the Derivatives of the Food Additive Fumaric Acid.Front Microbiol. 2018 May 17;9:896. doi: 10.3389/fmicb.2018.00896. eCollection 2018. Front Microbiol. 2018. PMID: 29867807 Free PMC article.
-
Malakoplakia mimicking malignant rectal carcinoma with bone erosion in a SLE patient: diagnosis insights from a case report and literature review.Front Immunol. 2025 May 21;16:1542777. doi: 10.3389/fimmu.2025.1542777. eCollection 2025. Front Immunol. 2025. PMID: 40469298 Free PMC article. Review.
-
Nucleoside Analogues as Antibacterial Agents.Front Microbiol. 2019 May 22;10:952. doi: 10.3389/fmicb.2019.00952. eCollection 2019. Front Microbiol. 2019. PMID: 31191461 Free PMC article. Review.
-
Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease.Front Pharmacol. 2021 Jul 14;12:684486. doi: 10.3389/fphar.2021.684486. eCollection 2021. Front Pharmacol. 2021. PMID: 34335253 Free PMC article. Review.
-
Interplay between inflammatory bowel disease therapeutics and the gut microbiome reveals opportunities for novel treatment approaches.Microbiome Res Rep. 2023 Sep 26;2(4):35. doi: 10.20517/mrr.2023.41. Microbiome Res Rep. 2023. PMID: 37849974 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

