Regulation of the Functions of Natural Cytotoxicity Receptors by Interactions with Diverse Ligands and Alterations in Splice Variant Expression
- PMID: 28424697
- PMCID: PMC5371597
- DOI: 10.3389/fimmu.2017.00369
Regulation of the Functions of Natural Cytotoxicity Receptors by Interactions with Diverse Ligands and Alterations in Splice Variant Expression
Abstract
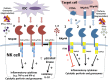
The natural cytotoxicity receptor (NCR) family is constituted by NKp46, NKp44, and NKp30 in humans, which are expressed mainly on natural killer (NK) cells and are encoded by the ncr1, ncr2, and ncr3 genes, respectively. NCRs have classically been defined as activating receptors that trigger cytotoxicity and cytokine responses by NK cells upon engaging with ligands on tumor cells. Several new findings, however, have challenged this model and identified alternative mechanisms regulating the function of NCRs. Recent reports indicate that ligand matters, since the interaction of NKp44 with distinct ligands on target cells can either activate or inhibit NK cells. Also, the NCRs have been found to interact with distinct specificities to various heparan sulfate glycosaminoglycans, which are complex polysaccharides found in extracellular matrix or on cell surface heparan sulfate proteoglycans (HSPGs). The NCRs can engage with HSPGs in trans as a co-ligand on the target cells or in cis on the NK cell surface to regulate receptor-ligand interactions and NK cell activation. A number of splice variants of ncr2 and ncr3 have also been identified, and a predominant expression of certain variants results in inhibitory signaling through NKp44 and NKp30. Several recent studies have found that the selective expression of some of these inhibitory splice variants can significantly influence outcome in the contexts of cancer, infection, and pregnancy. These findings establish that NCR functions are more diverse than originally thought, and better understanding of their splice variant expression profiles and ligand interactions are needed to establish their functional regulation in the context of human health.
Keywords: RNA splice variants; cancer immunology; cytotoxicity; human immunology; natural cytotoxicity receptors; natural killer cells; pregnancy; virus immunity.
Figures

References
-
- MacFarlane AW, 4th, Campbell KS. Signal transduction in natural killer cells. Curr Top Microbiol Immunol (2006) 298:23–57. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

